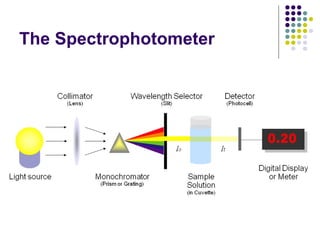
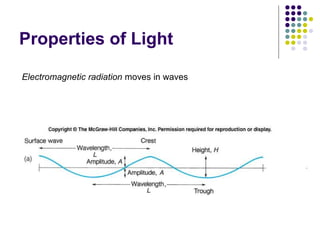
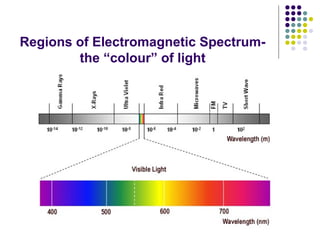
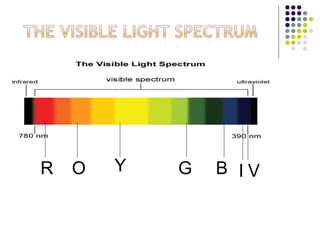
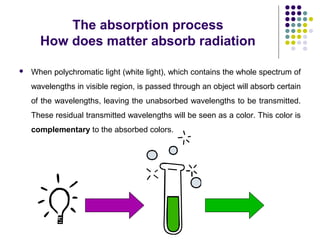
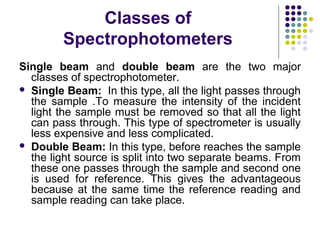
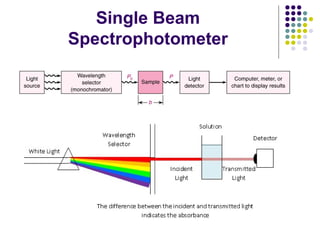
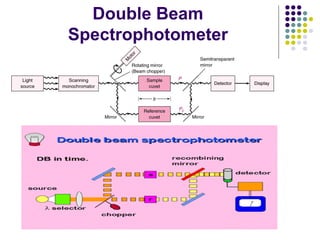
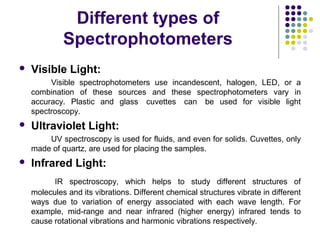
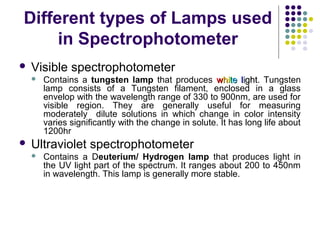
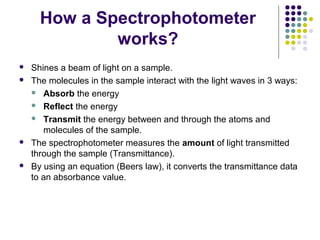
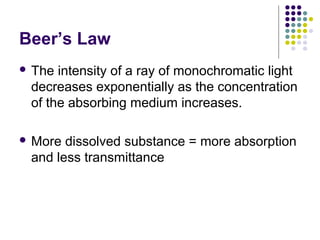
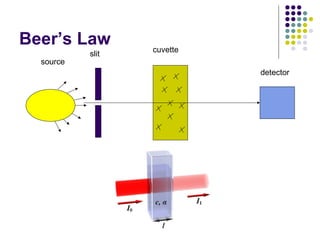
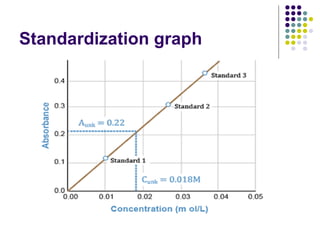
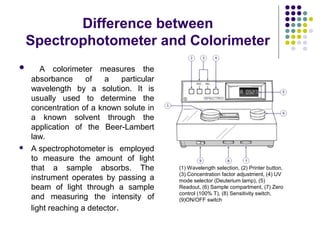
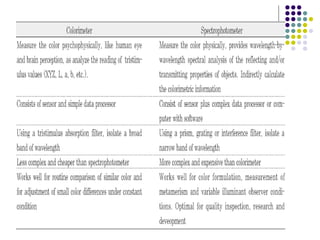
Spectrophotometry involves using a spectrophotometer to measure how much light is absorbed or transmitted by a sample at different wavelengths. It is commonly used to determine the concentration of known substances in solution. A spectrophotometer works by shining light through a sample and measuring the intensity of the transmitted light, then using Beer's Law to calculate absorbance from transmittance. Absorbance measurements at different concentrations can be used to generate a standard curve to determine the concentration of unknown samples. Spectrophotometers are versatile tools that are widely used in laboratory analysis across various industries.