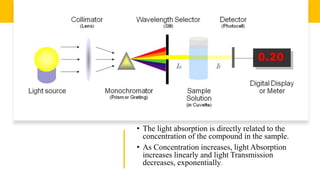
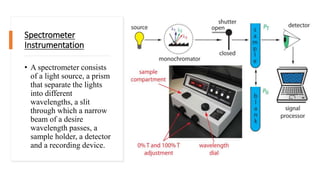
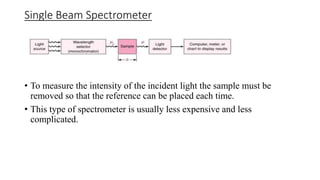
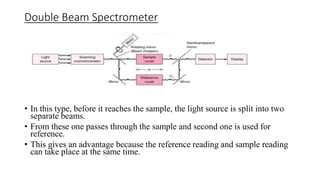
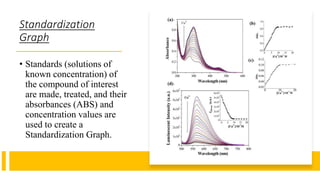
Spectrophotometry uses light absorption properties of substances to quantitatively analyze samples. It follows Beer's Law, where absorbance is directly proportional to concentration. A spectrophotometer splits light into wavelengths, passes a sample beam through the sample, and measures the intensities of light transmitted versus a reference beam. This allows measurement of absorbance across wavelengths. Main applications include concentration measurement, detection of impurities, and studying chemical kinetics.