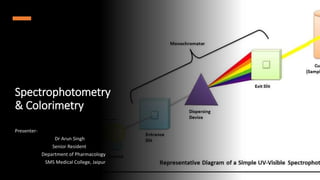
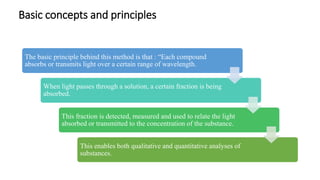
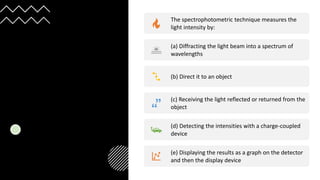
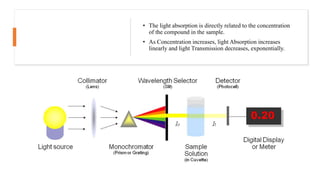
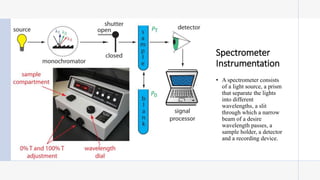
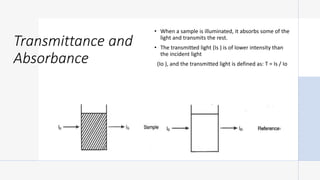
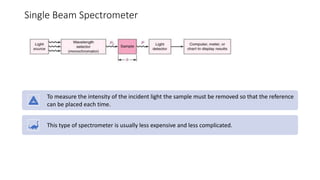
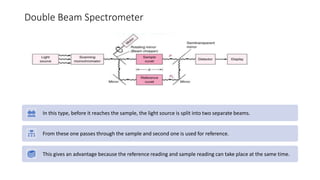
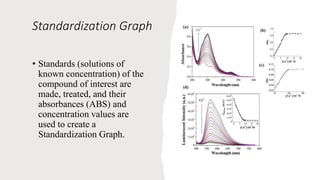
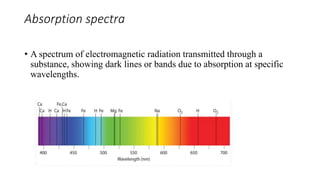
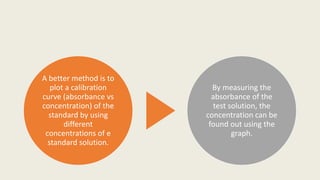
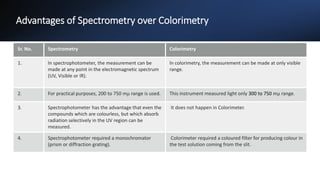
This document provides an overview of spectrophotometry and colorimetry. It discusses the basic principles including how spectrophotometry follows Beer's law and relates light absorption to sample concentration. It describes the history and development of spectrophotometry instrumentation. The basic components and mechanisms of spectrophotometers are outlined. Applications of spectrophotometry include concentration measurement, detection of impurities, and molecular weight determination. Colorimetry is similar but uses only the visible light range. Spectrophotometry has advantages over colorimetry in being able to measure a broader electromagnetic spectrum.