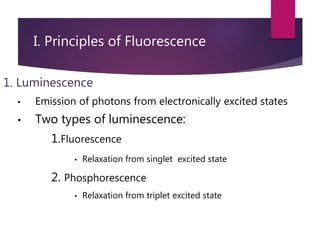
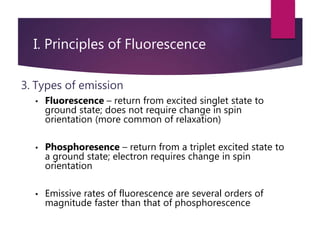
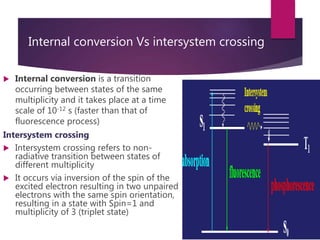
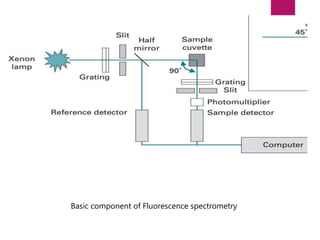
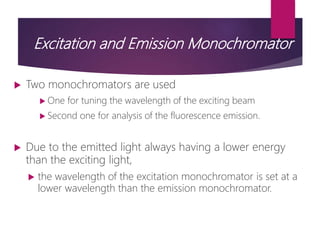
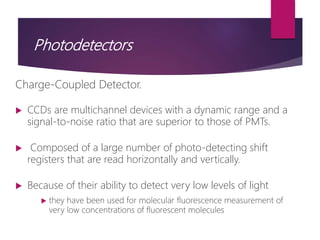
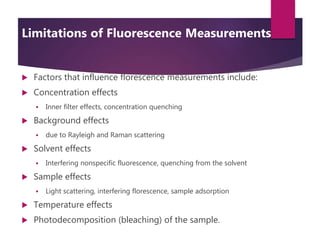
The document discusses spectrofluorometry, focusing on the principles of fluorescence, the types of emission, and the instrumentation involved in fluorescence measurement. It outlines the interaction of light with molecules, types of fluorophores, and the techniques for measuring fluorescence intensity related to concentration using the Beer-Lambert Law. Additionally, it highlights the advantages, limitations, and applications of fluorescence spectroscopy in fields like environmental science, analytical chemistry, medicine, and pharmacy.