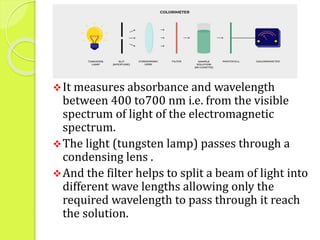
Colorimetry uses the light absorbing properties of solutions to measure concentration. It follows Beer's and Lambert's laws - the amount of light absorbed is directly proportional to the concentration and path length of the solution. The photoelectric colorimeter is commonly used to measure substances in blood and body fluids. It has advantages like being inexpensive and portable but cannot analyze colorless compounds or work in some light regions.