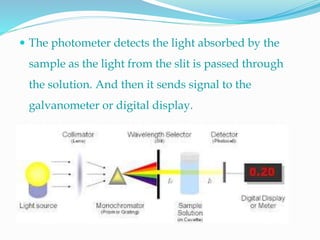
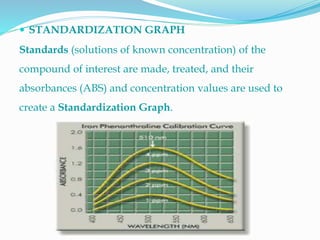
The document explains spectrophotometry, a method for quantitatively measuring the absorption of light by compounds, directly related to their concentration. It describes the working and components of spectrophotometers, which can be single or double beam, and categorized by the light wavelength they utilize (visible, UV, and IR). Additionally, it covers the Beer-Lambert Law, standardization processes, and various applications of spectrophotometry.