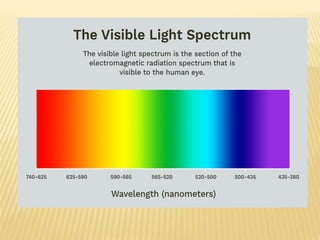
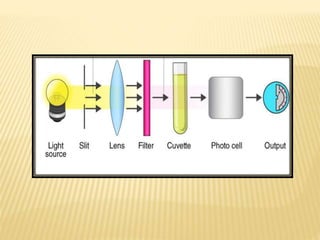
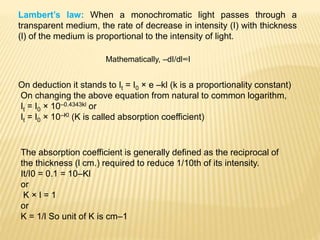
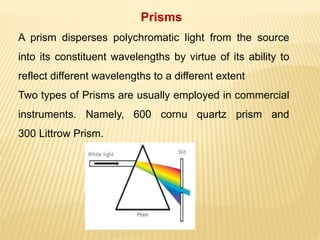
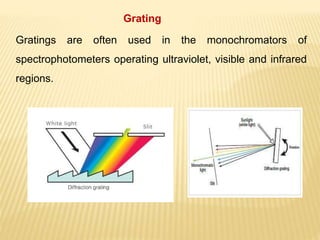
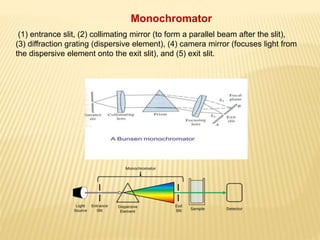
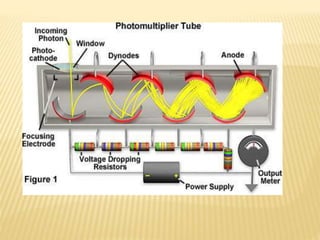
The document discusses visible spectrophotometry and colorimetry as analytical techniques for measuring light absorption in samples to determine solute concentration. It explains the principles behind spectrophotometry, including Lambert's and Beer's laws, detailing the components and functioning of instruments like spectrophotometers and colorimeters. Various filters, detectors, and their applications in determining chemical properties and concentrations are also outlined.