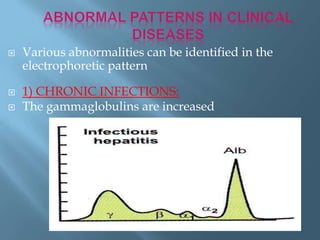
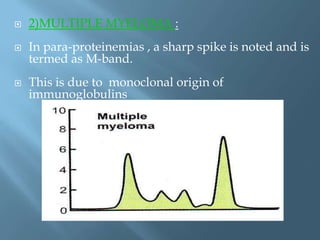
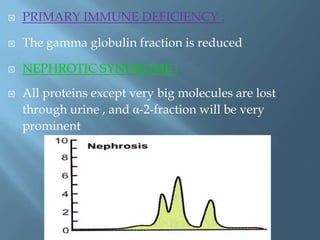
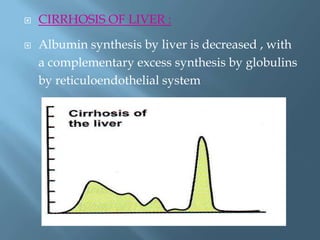
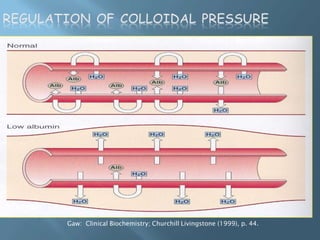
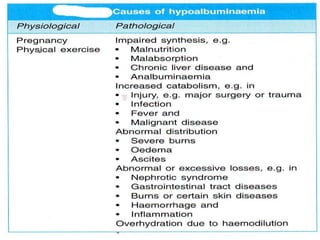
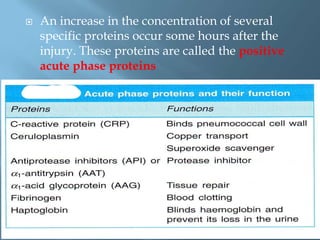
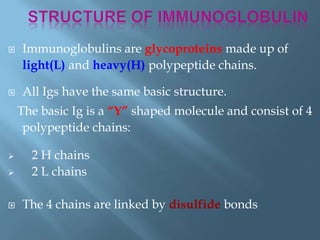
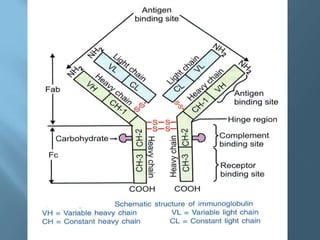
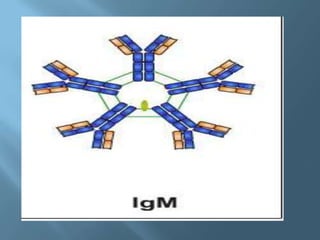
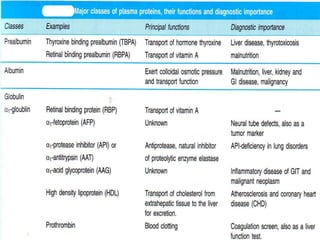
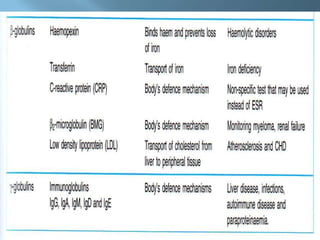
This document provides information on plasma and plasma proteins. It discusses that plasma constitutes 55% of blood volume and is composed mainly of water (91%) and proteins (8%). The major plasma proteins are albumin, globulins, and fibrinogen. Albumin makes up 60% of total plasma proteins. Various plasma proteins and their functions are described in detail. Abnormalities in plasma proteins can provide clinical information on diseases.