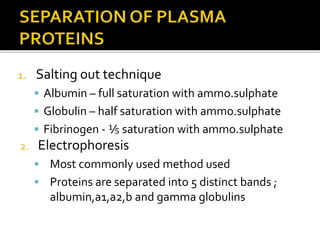
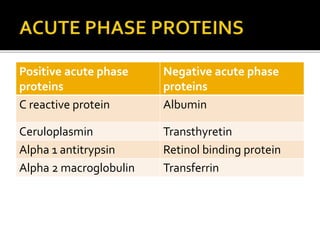
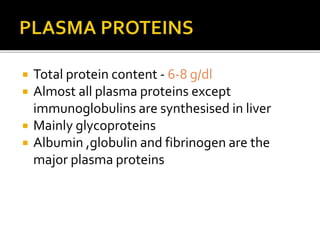
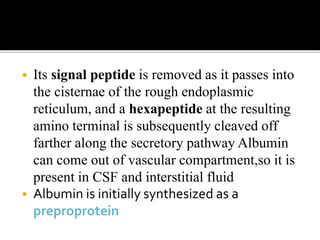
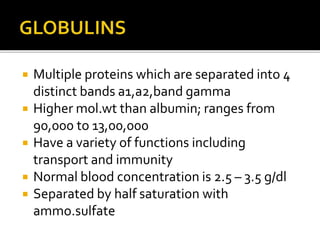
The document discusses several major plasma proteins including albumin, globulins, fibrinogen, and acute phase proteins. Albumin is the most abundant plasma protein synthesized by the liver and helps maintain blood volume and transport substances through the bloodstream. The main globulin proteins are alpha, beta, and gamma globulins which have roles in transport and immunity. Fibrinogen aids in blood clotting. C-reactive protein and ceruloplasmin are acute phase proteins that increase during inflammation.


![ Predominant plasma protein,water soluble
Synthesised in liver
The name is derived from the white
precipitate formed when egg is boiled[albus
means white in Latin]
Normal blood level is 3.5-5 g/dl
Migrates fastest in electrophoresis at alkaline
pH and precipitates last in salting out
methods](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-5-320.jpg)
![ Structure and synthesis
Has one polypeptide chain consisting of 585
AAs with 17 disulfide bonds
Mol wt :69 KD,relatively low mol.wt
Daily production:12g/day
Estimation of albumin is a component of
liver function test [LFT]
Has a PI of 4.7](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-6-320.jpg)


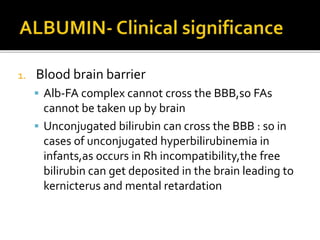
![ In hypo albuminemia,[decreased blood levels
of albumin] the EOP is correspondingly
decreased return of water to blood vessels
decreased accumulation/retention of water
in tissues EDEMA
Edema usually occurs when blood albumin
level is < 2g/dl](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-10-320.jpg)






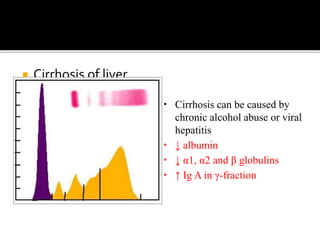
![5. Hypo albuminemia and edema
a. Malnutrition - albumin synthesis is depressed
[generalised edema]
b. Nephrotic syndrome - albumin is lost through
urine facial edema]
c. Cirrhosis of liver – decreased albumin synthesis
[ascites]
d. c/c congestive heart failure – pitting edema of
feet](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-18-320.jpg)



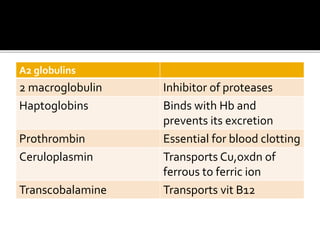
![Α1-globulins
A1 antitrypsin Inhibitor of trypsin and other
serine proteases
Oroso mucoid Transports progesterone
A1 lipoproteins Transports cholesterol from
tissues to liver
AFP Principal fetal protein
Retinol binding protein [RBP] Transports retinol
Thyroxine binding globulin
[TBG]
Transports thyroxine
Cortisol binding globulin
[CBG]or transcortin
Transports cortisol and
corticosterol](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-23-320.jpg)
![B globulins
B lipoproteins [LDL] Transports cholesterol and
triglycerides to tissues
B2 microglobulin Used to test renal tubular
function
Transferrin Transports iron
Hemopexins Transports heme
Plasminogen Involved in fibrinolysis](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-25-320.jpg)
![ Gamma globulins
Eg: CRP [C reactive protein]
Non specific defence against infectious agents](https://image.slidesharecdn.com/plasmaproteins-170313093429/85/Plasma-proteins-26-320.jpg)