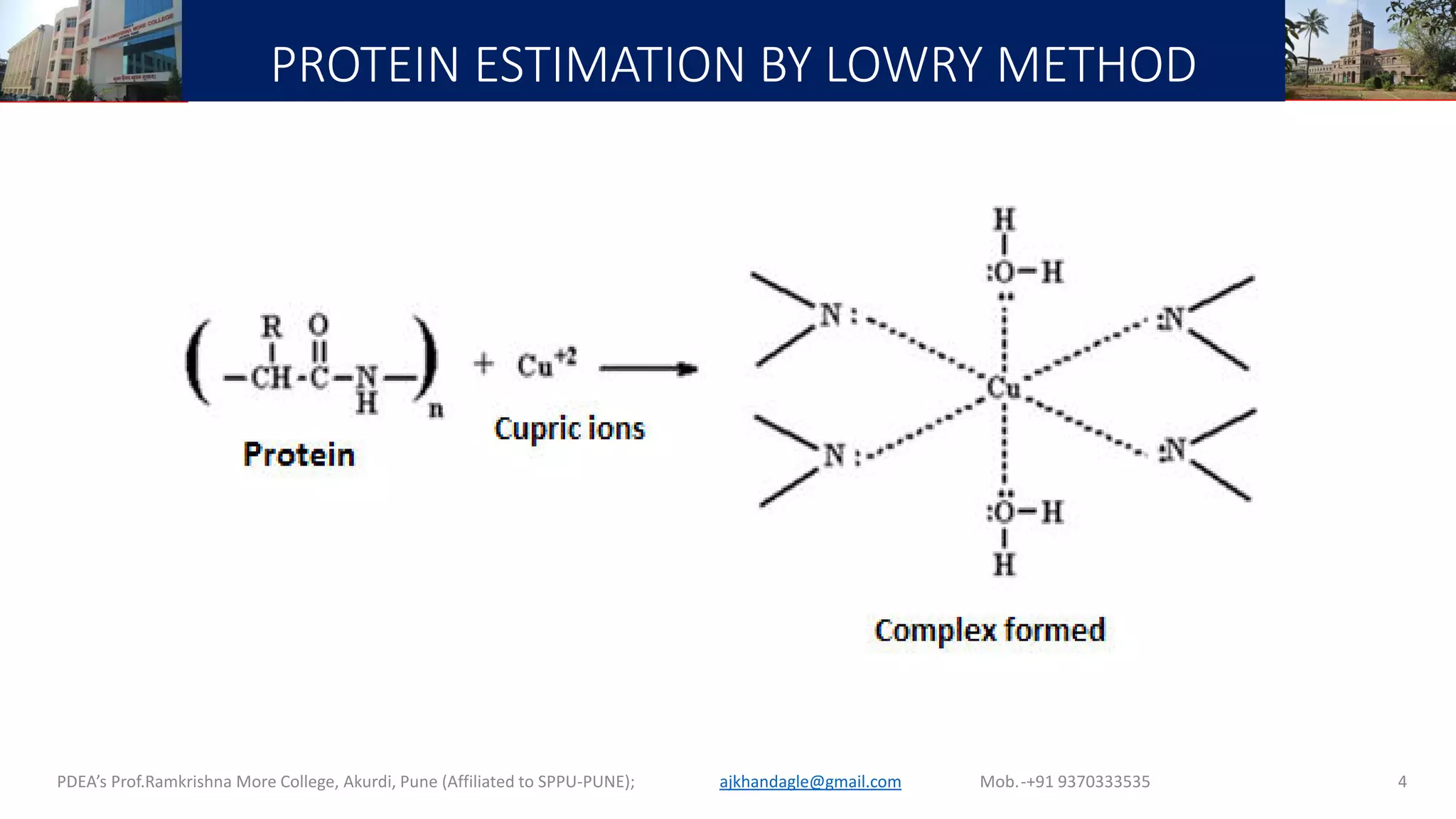
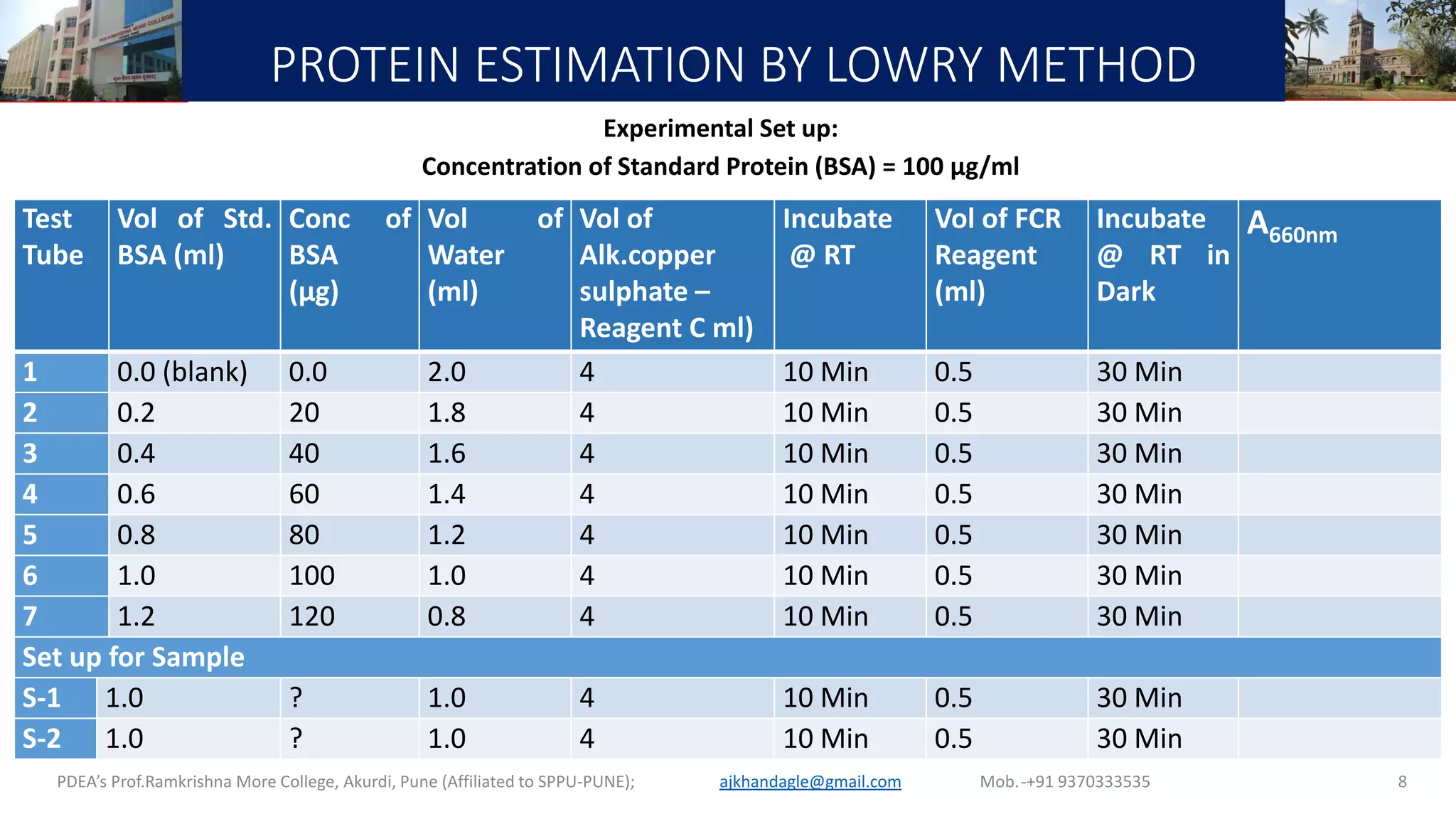
This document describes a laboratory experiment on estimating total protein concentration using the Lowry method. It involves preparing reagents, running a standard curve with bovine serum albumin (BSA) standards, and measuring absorbance to generate a calibration curve. Absorbance readings of unknown samples are then used to determine their protein concentrations based on the standard curve. Key steps include reacting protein samples with an alkaline copper tartrate solution and Folin reagent to produce a blue color complex, whose intensity is proportional to protein amount. The experiment aims to familiarize students with the Lowry method and generating standard curves for protein quantification.