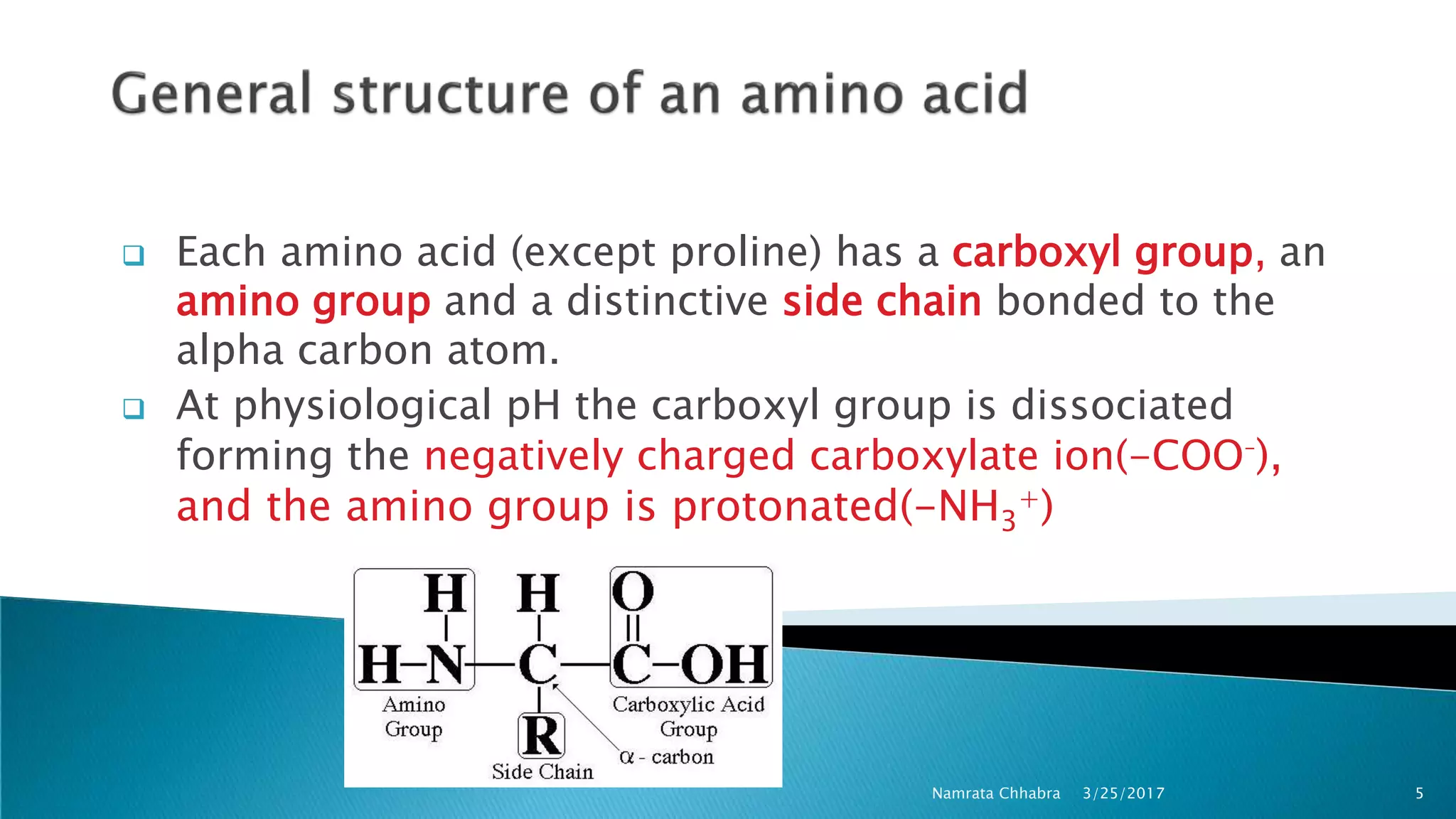
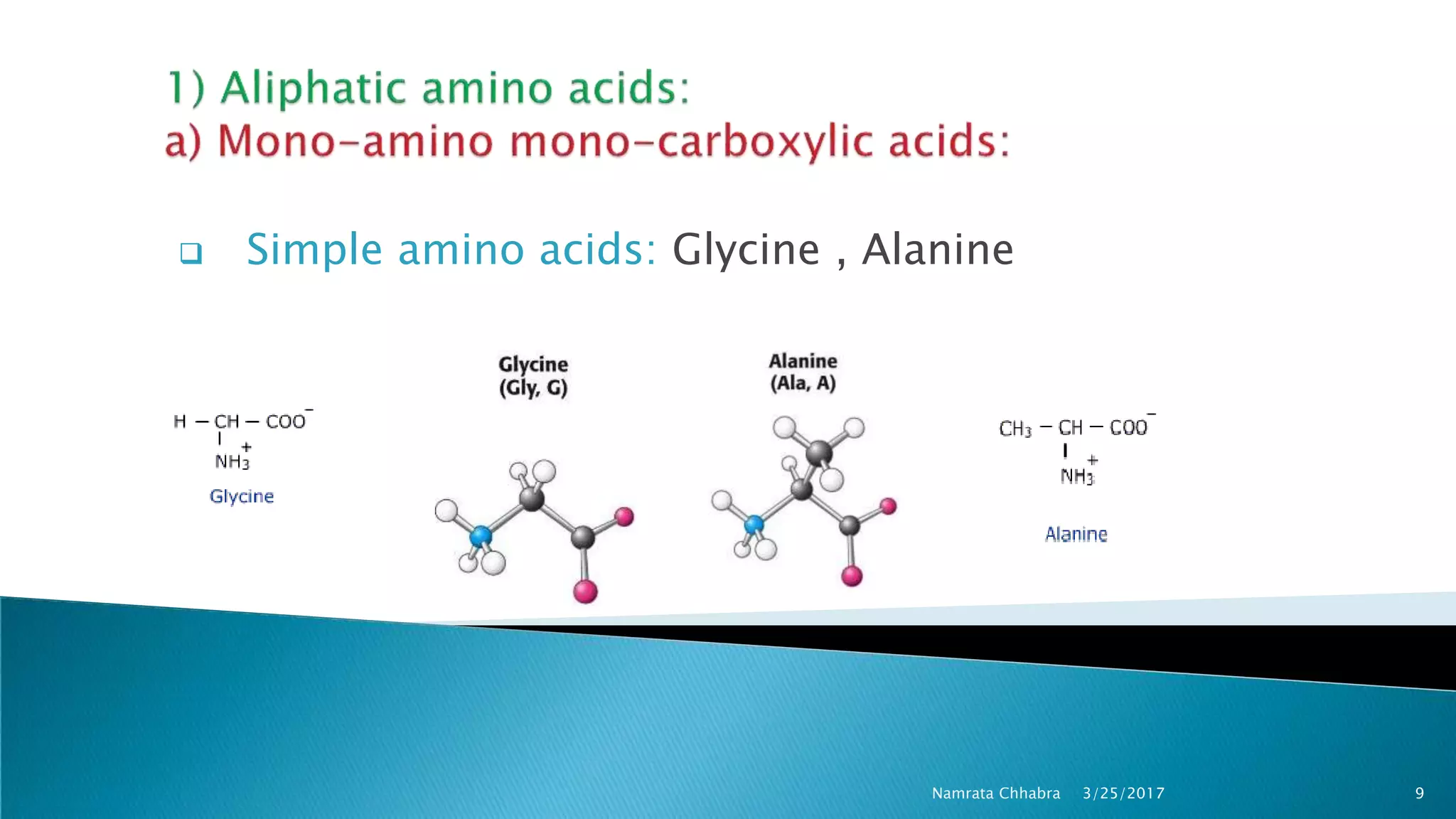
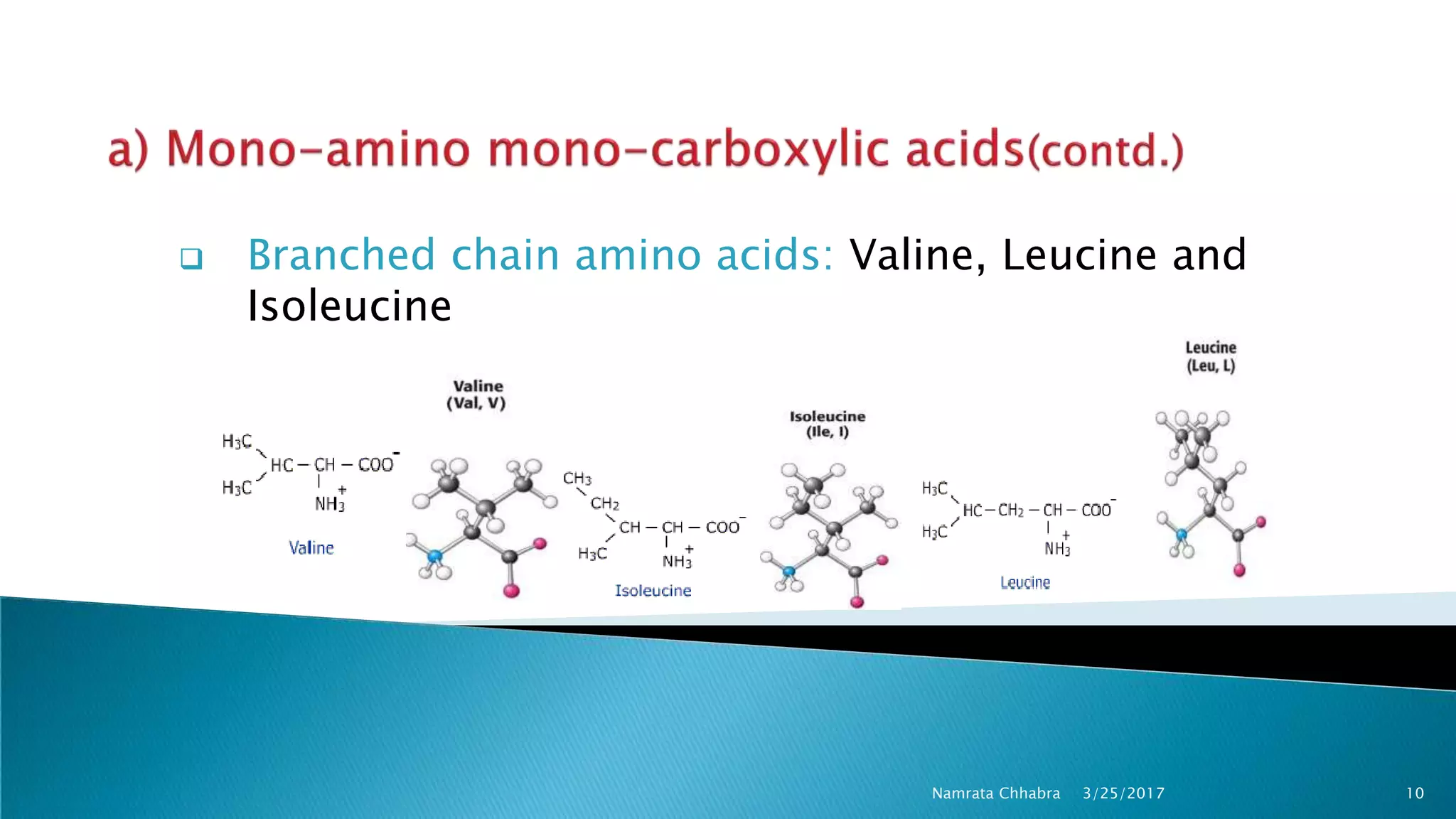
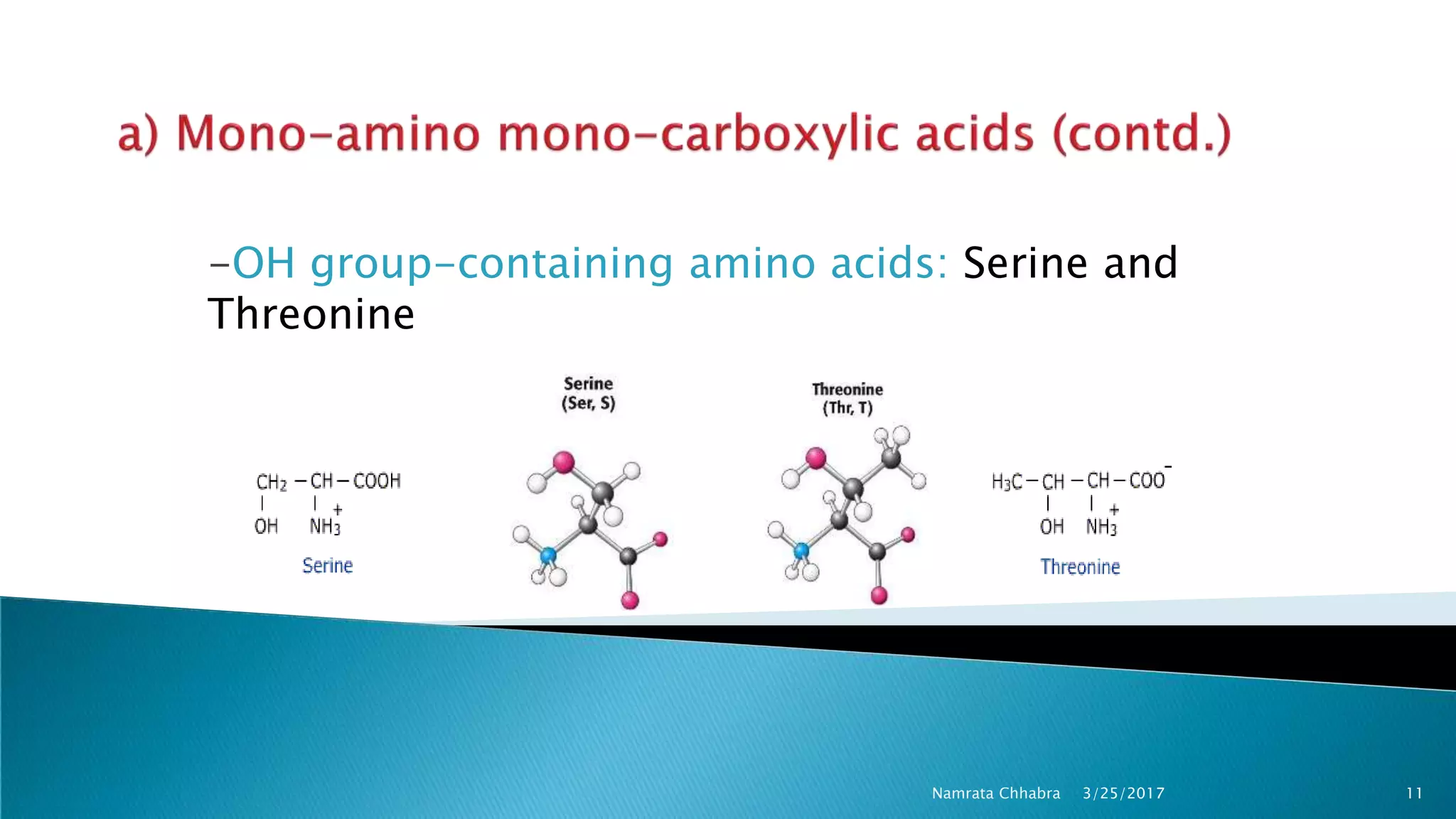
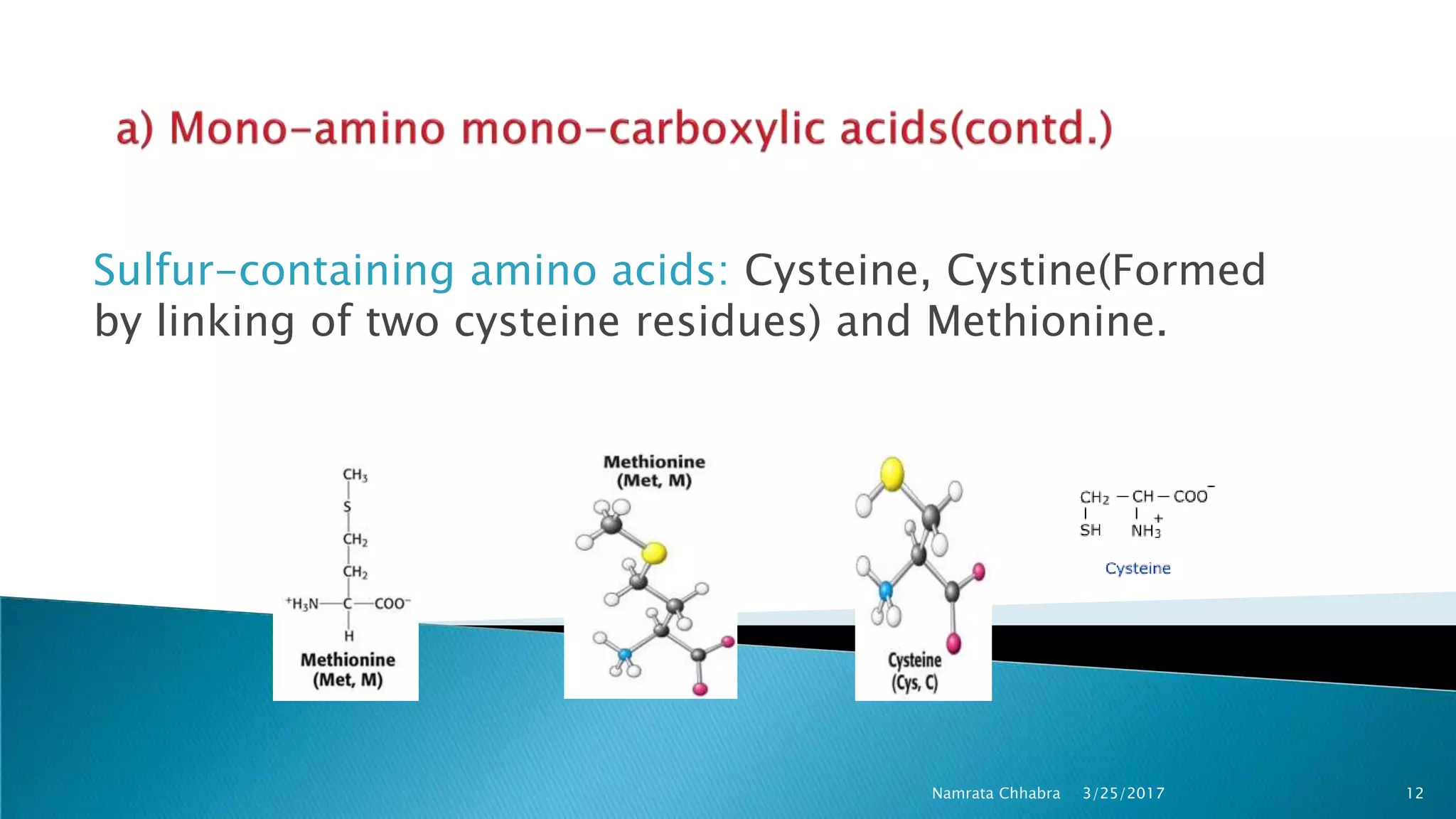
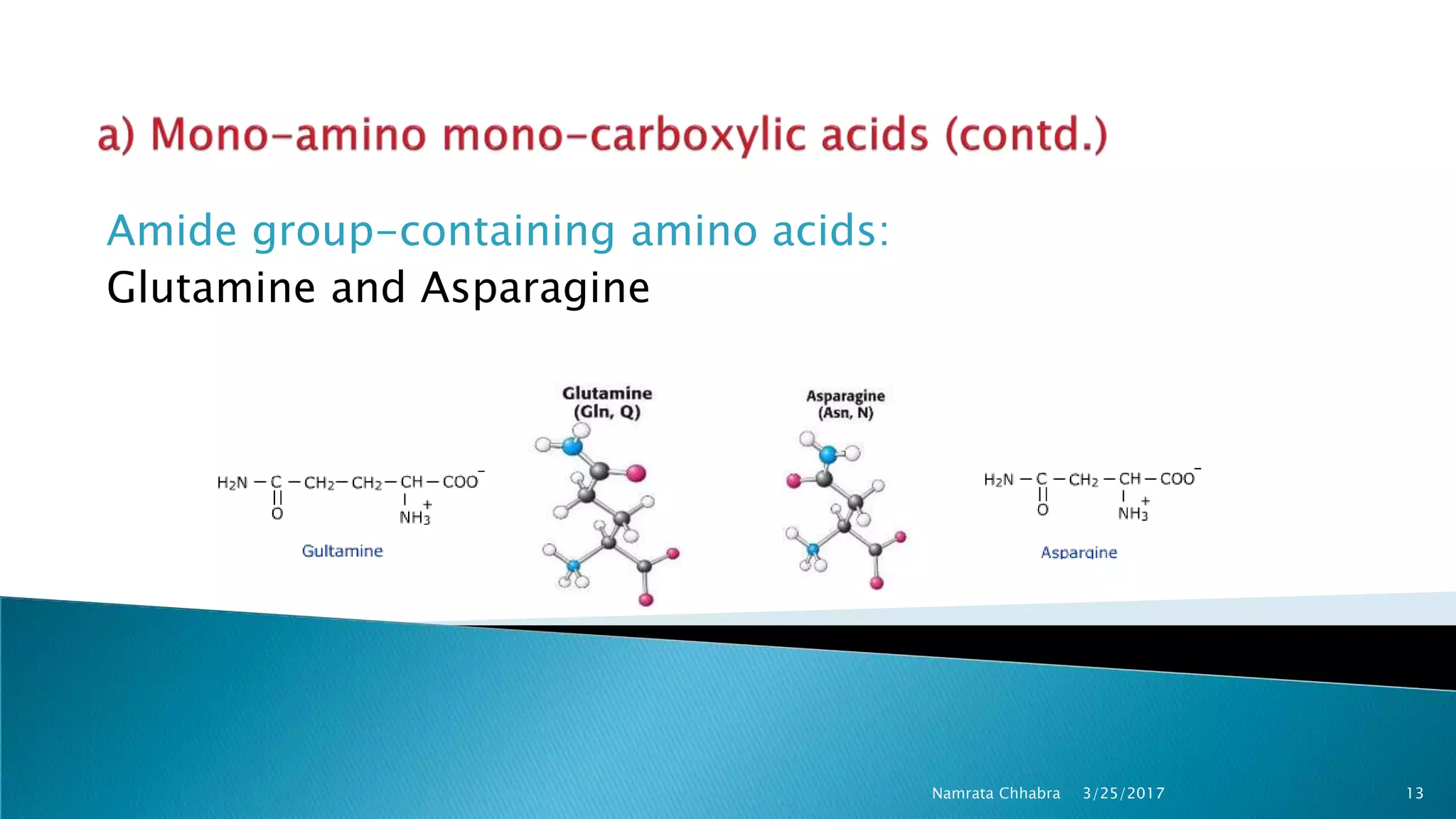
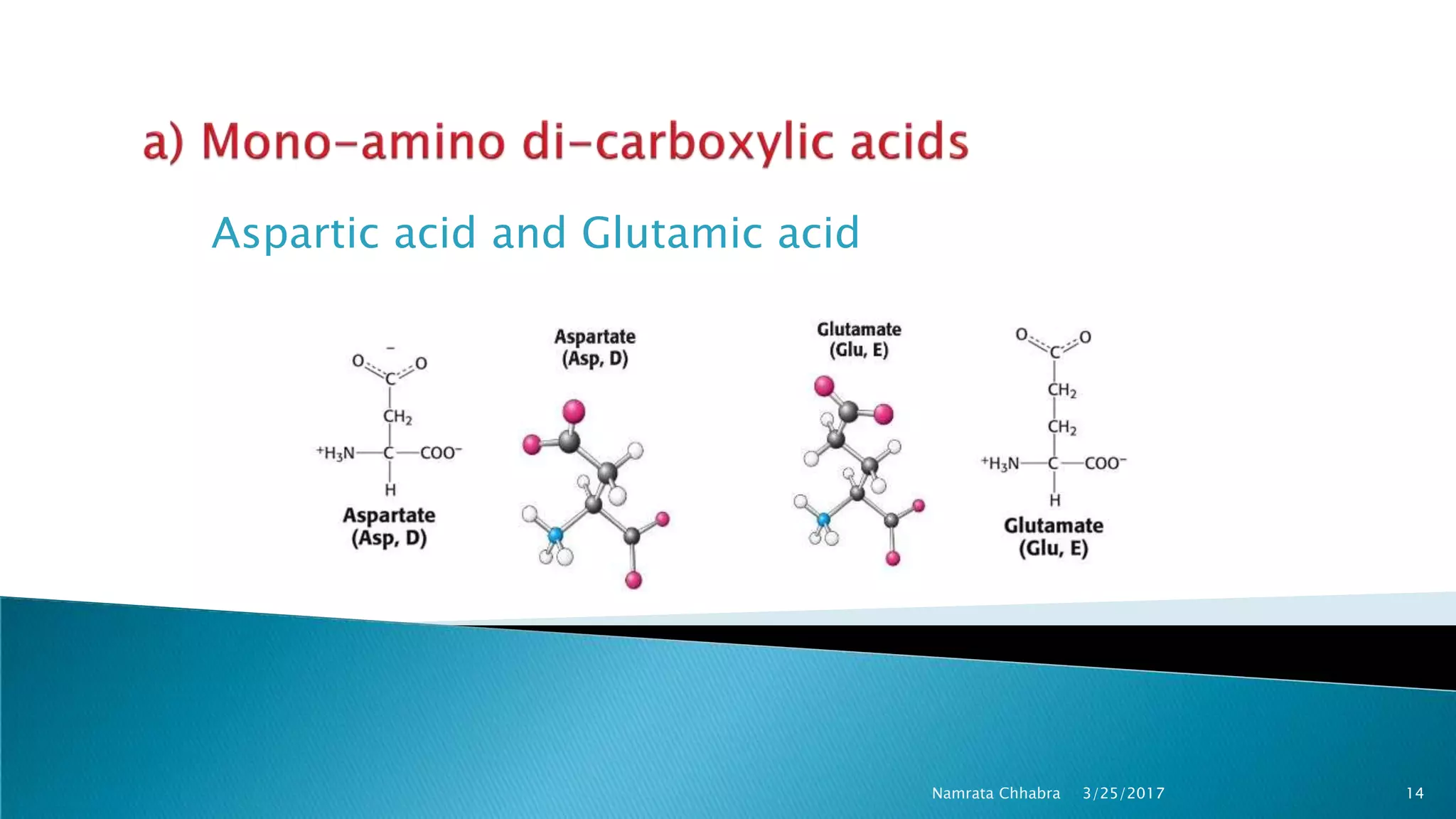
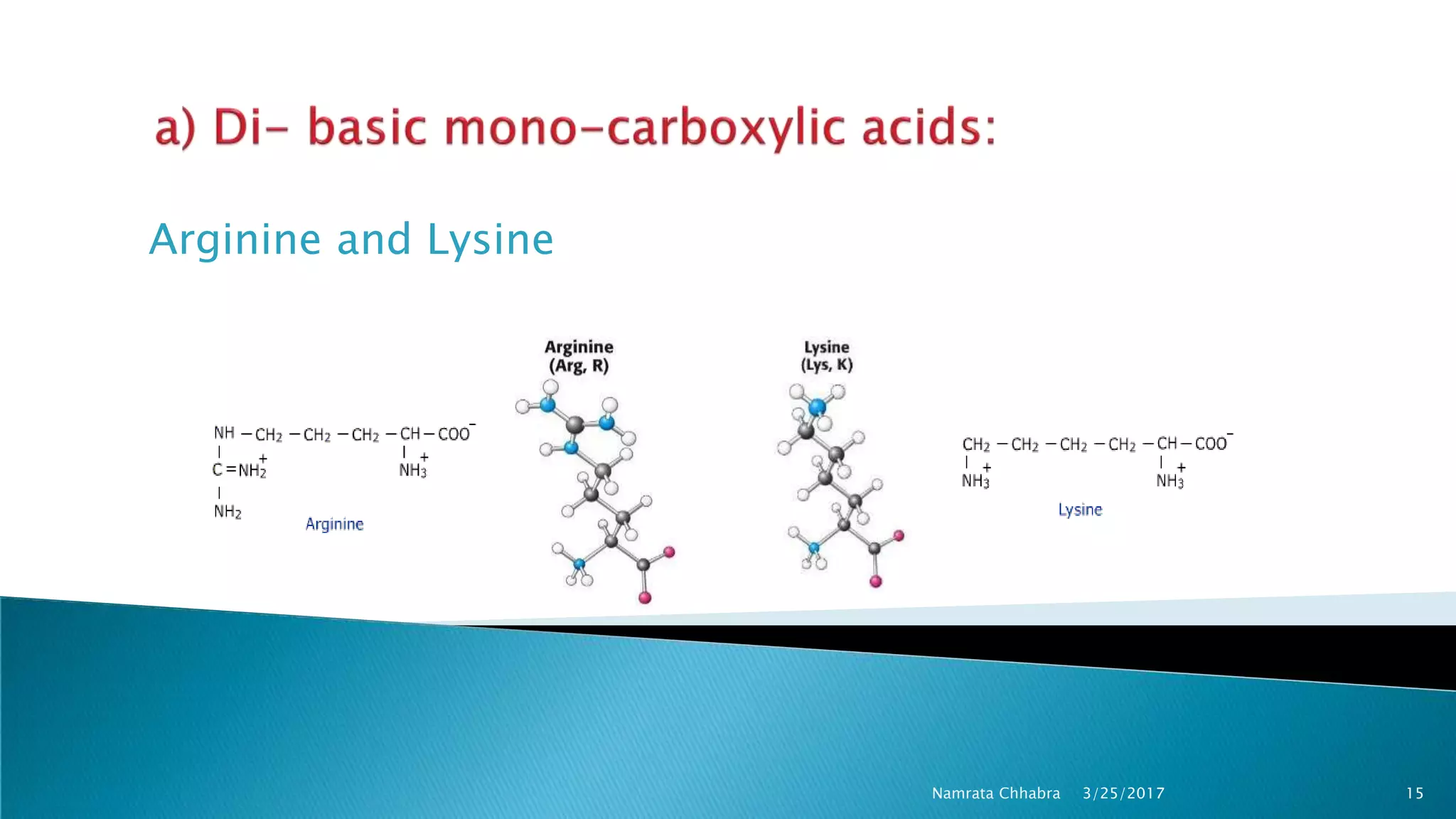
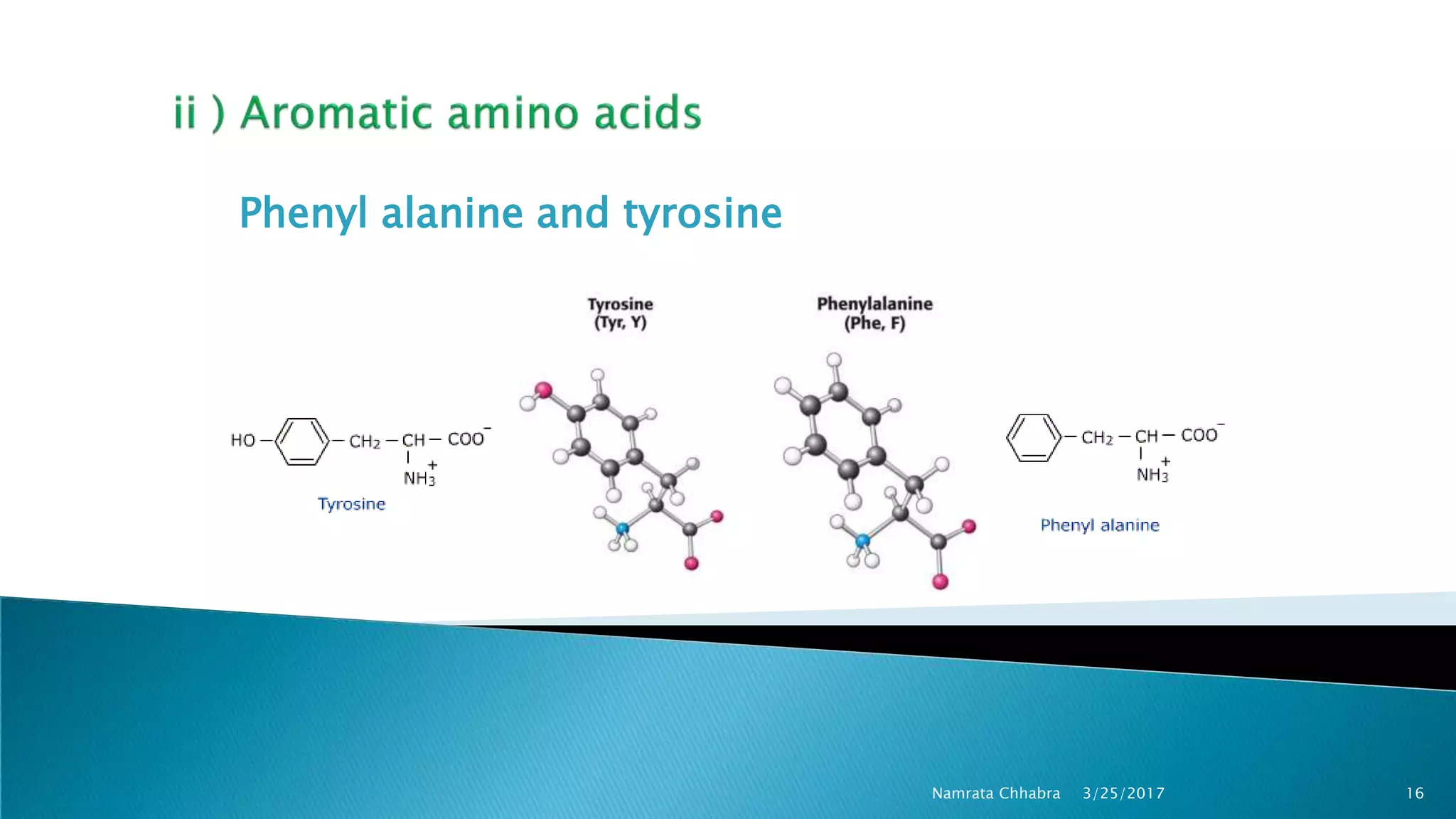
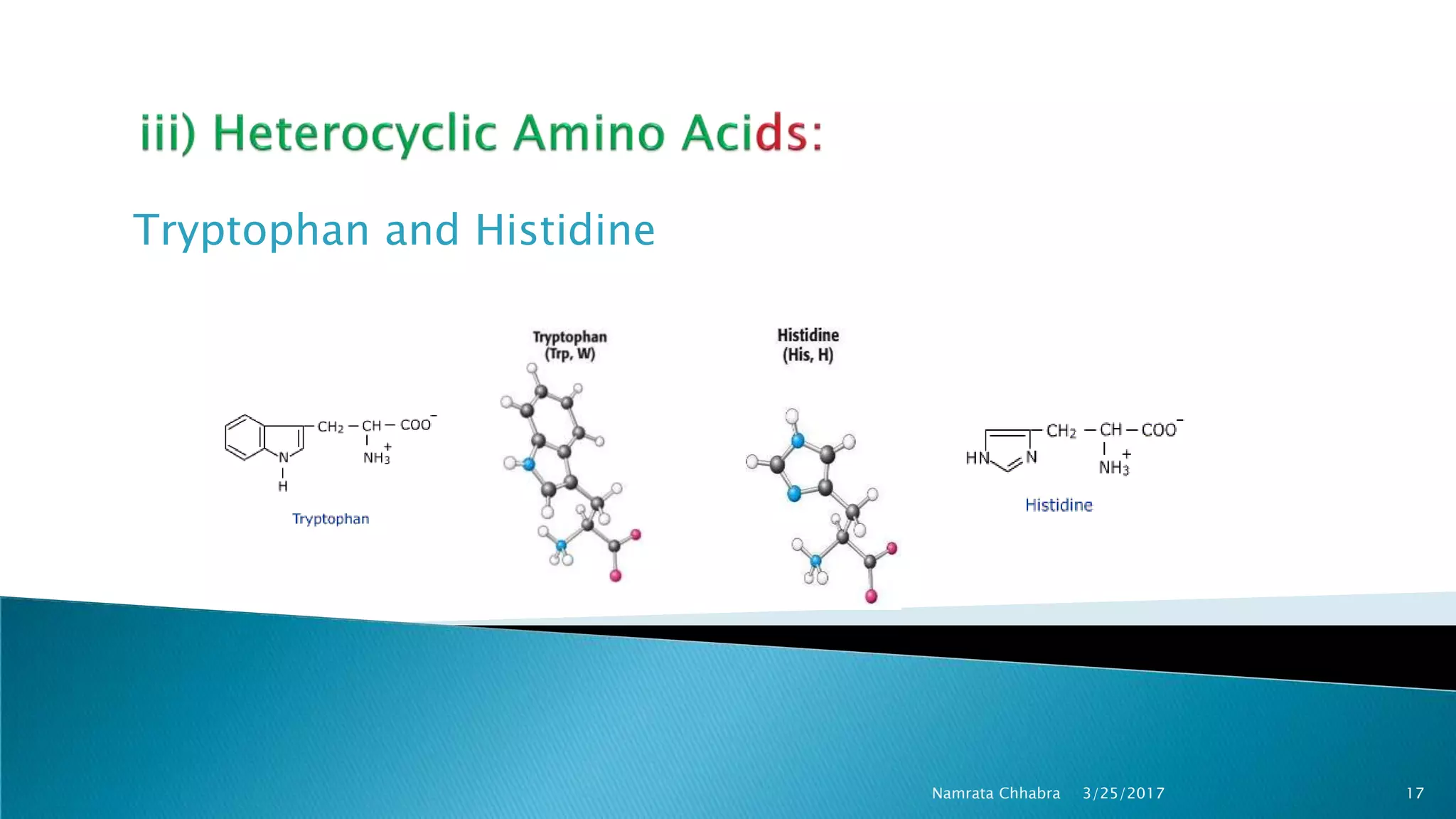
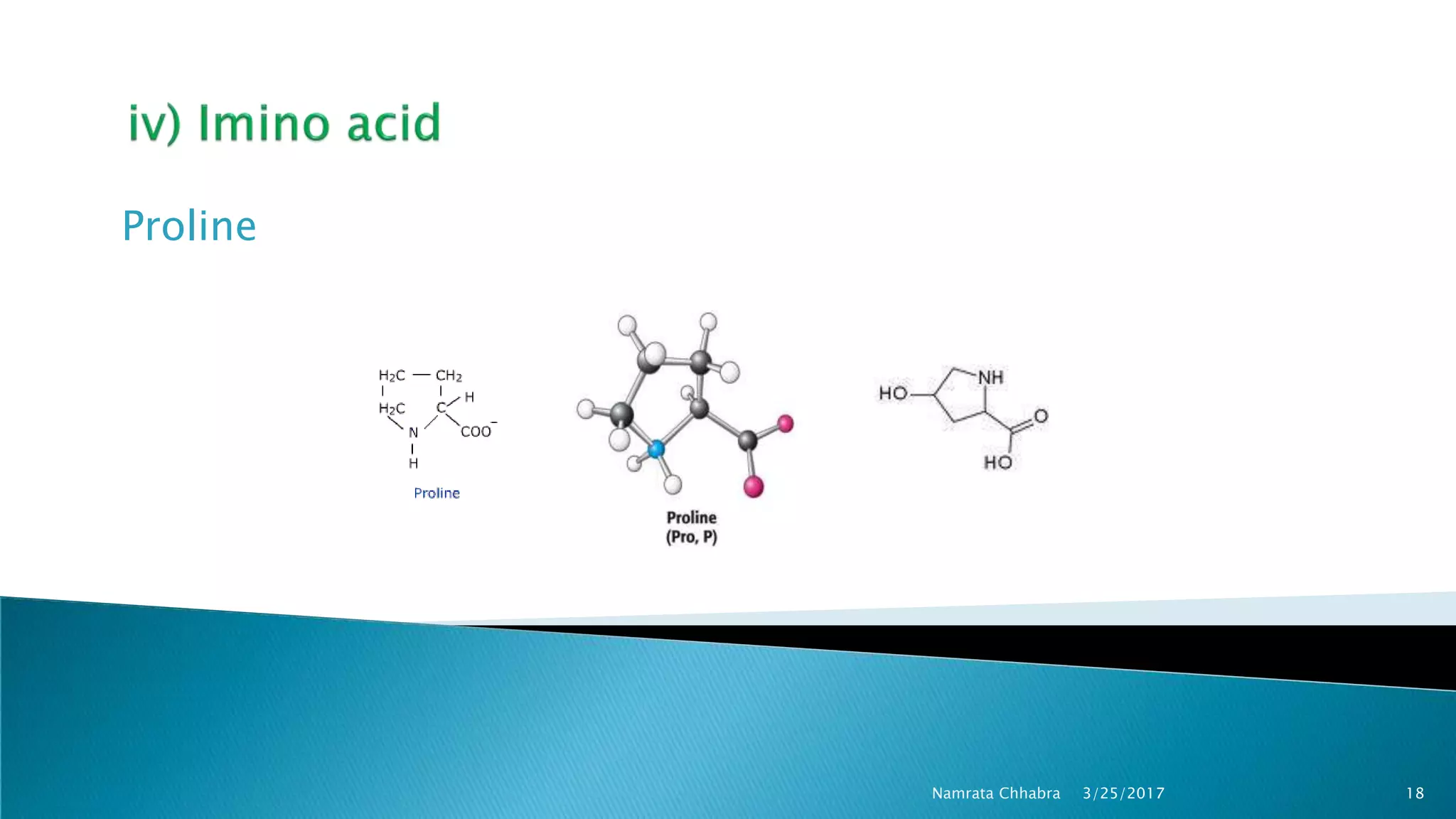
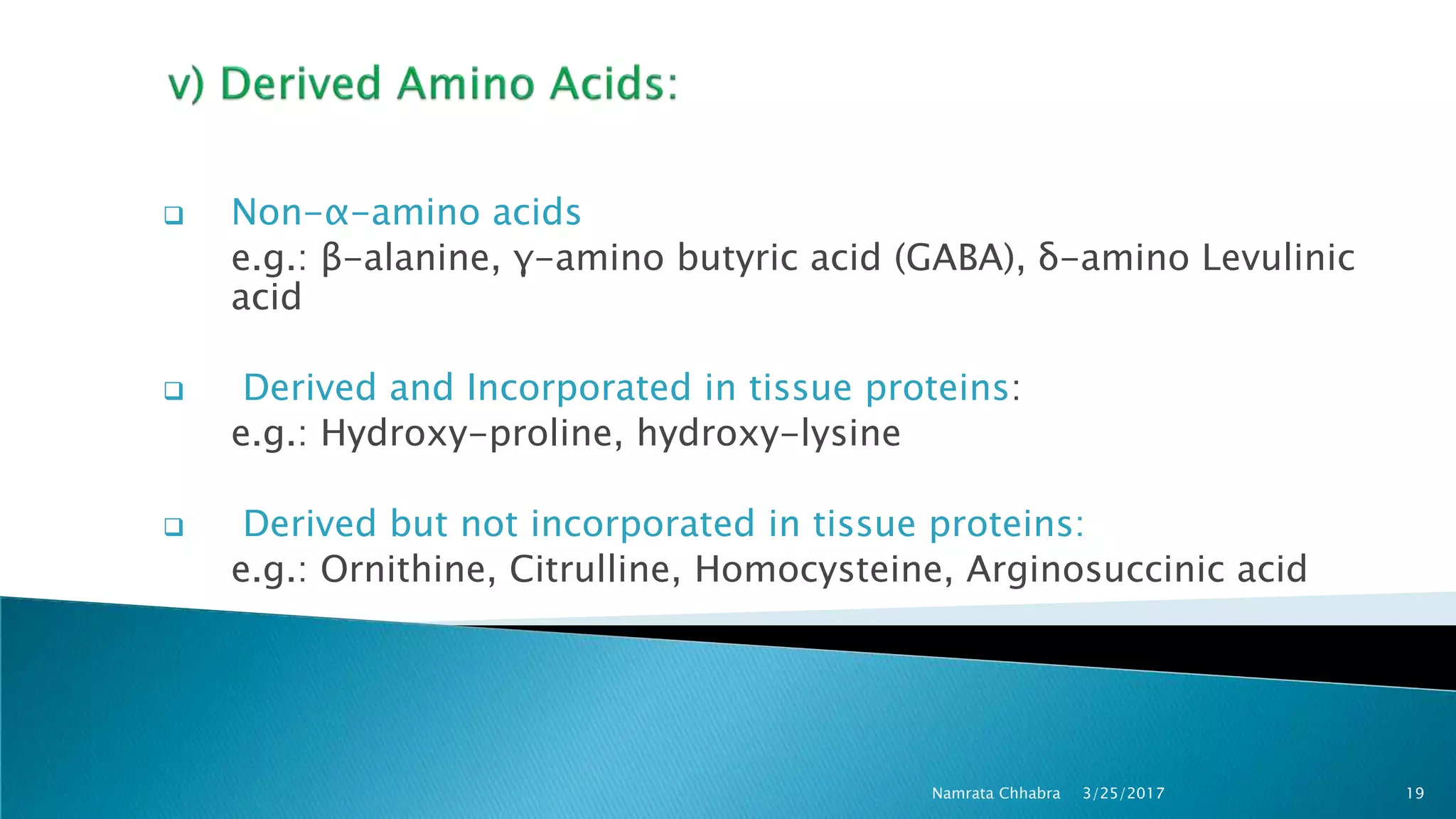
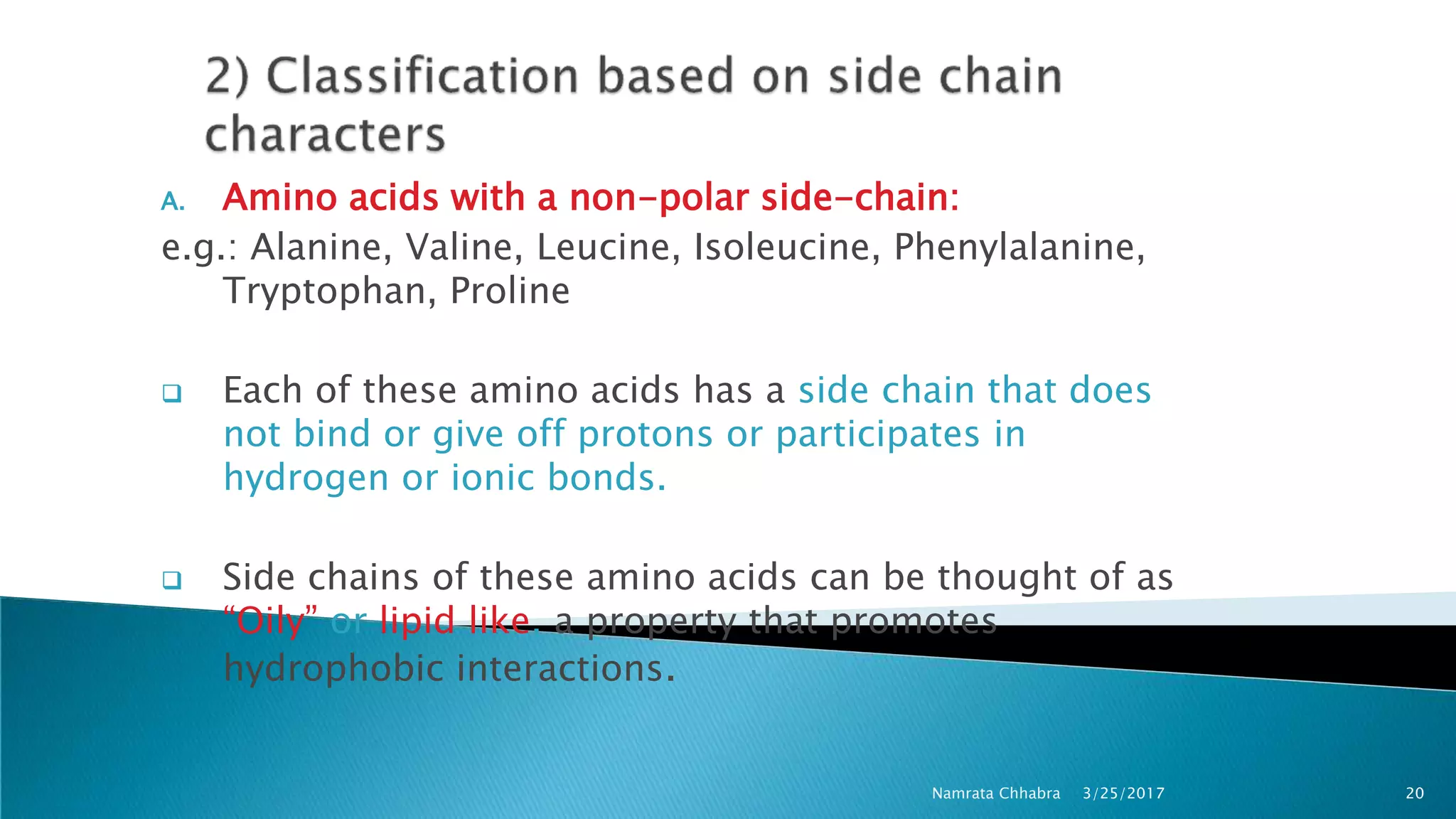
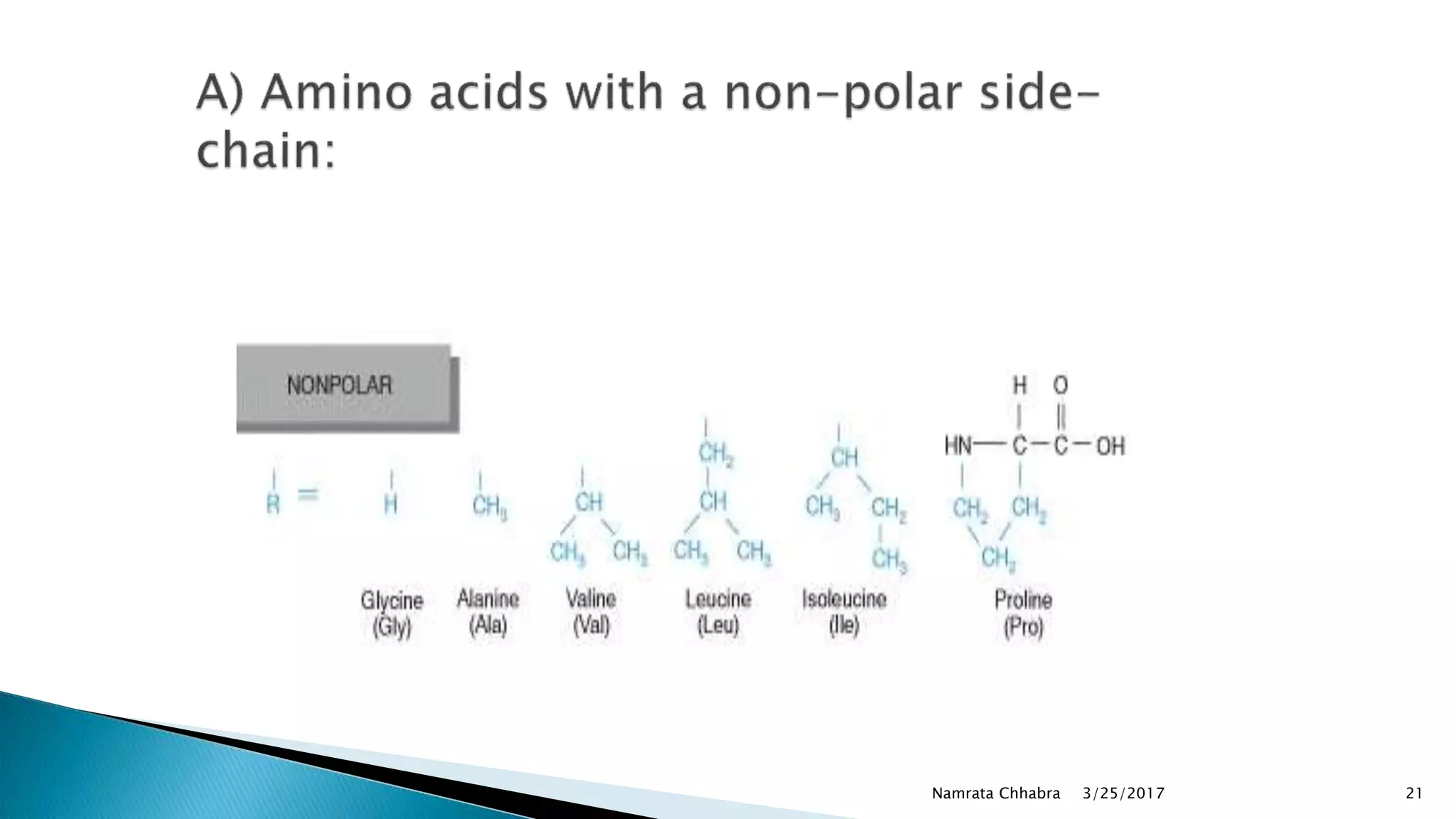
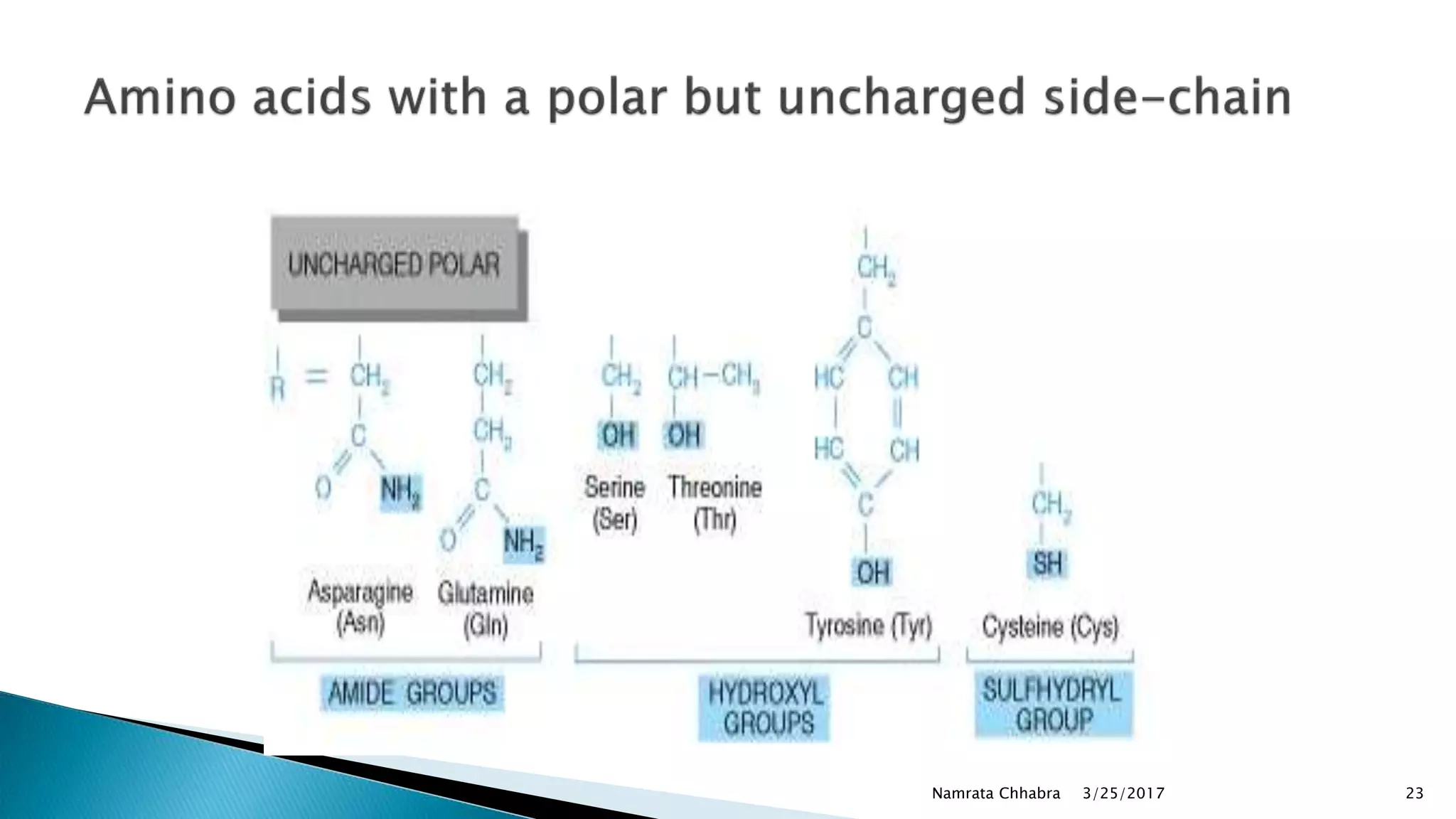
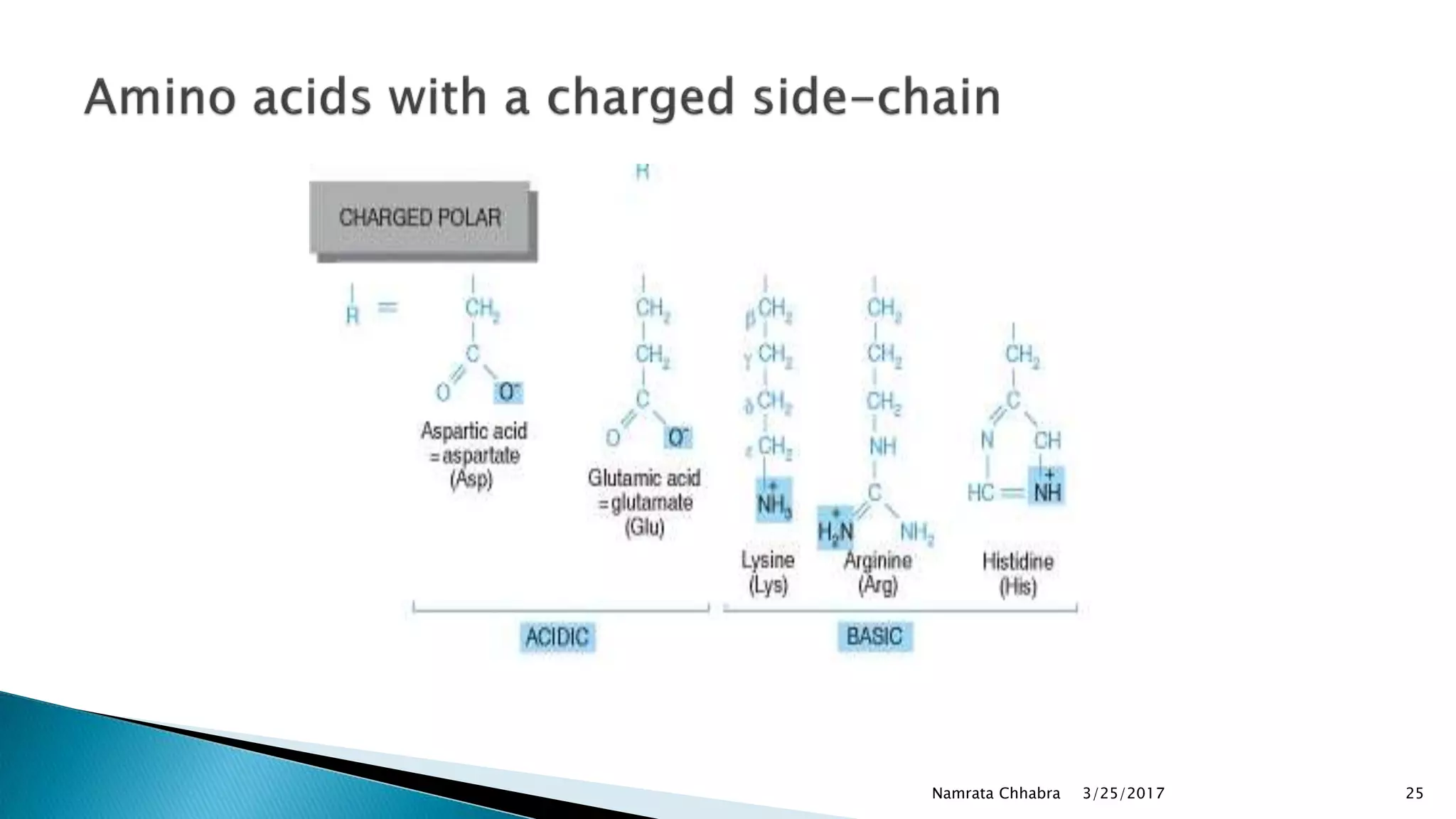
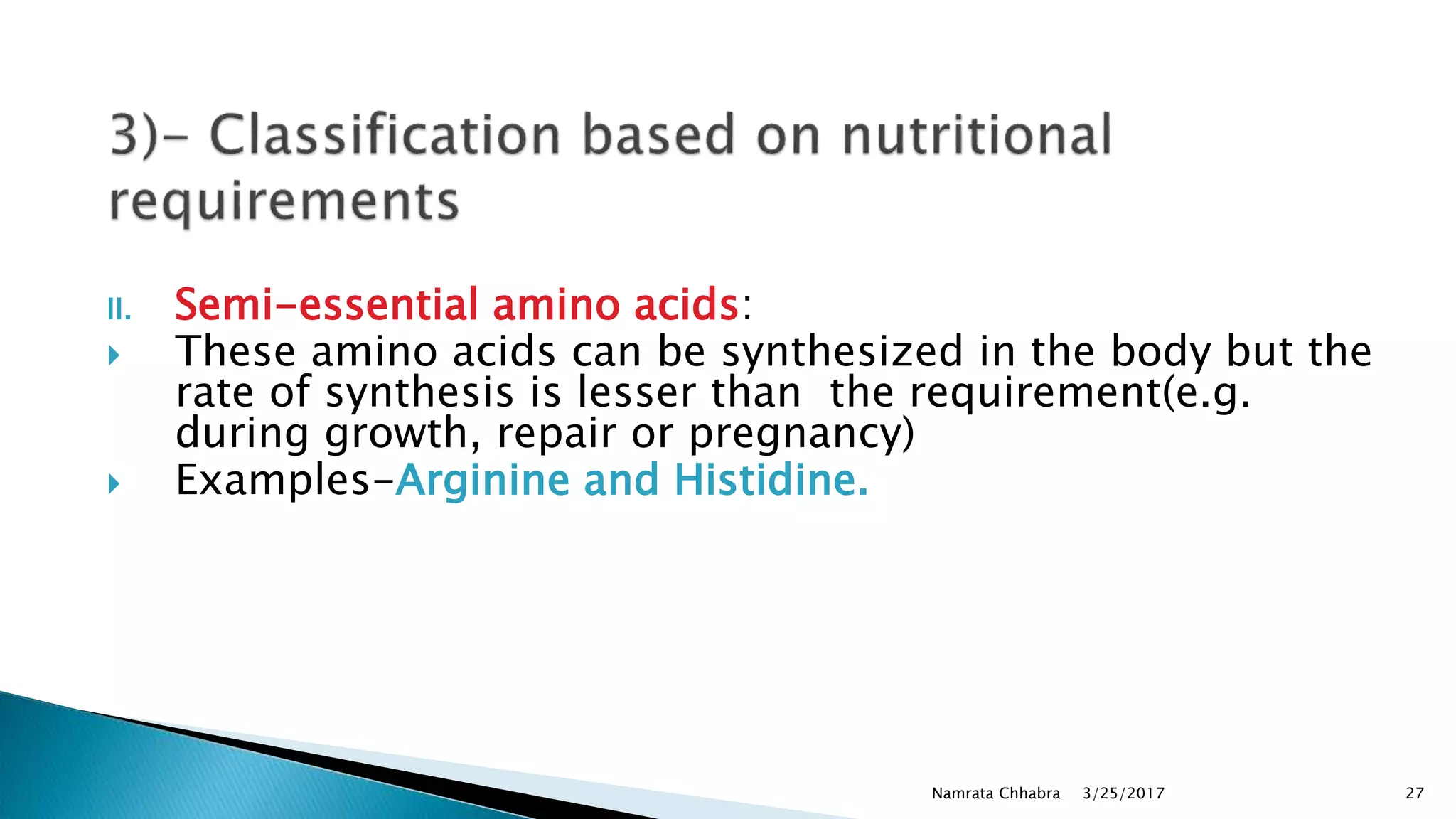
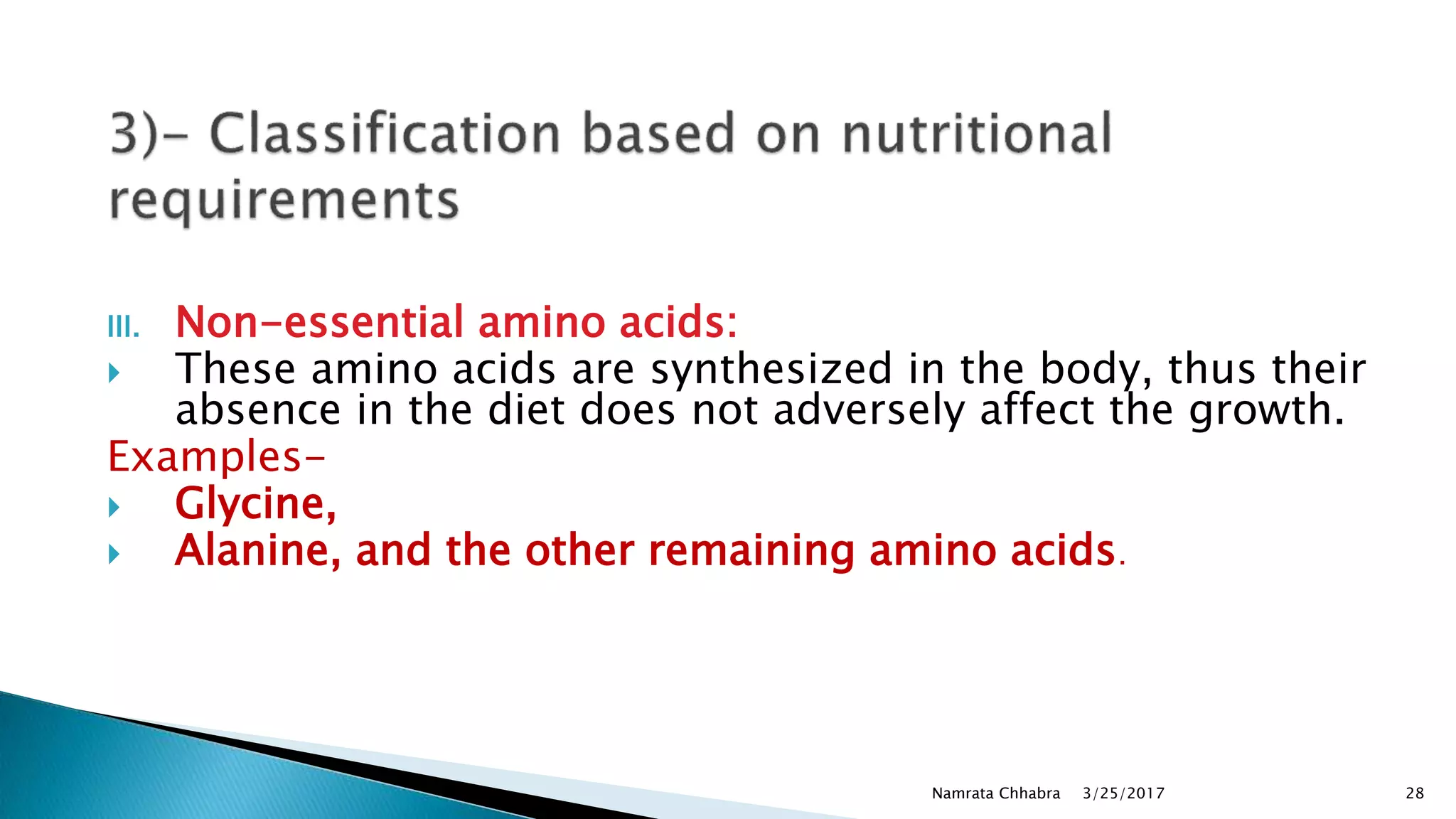
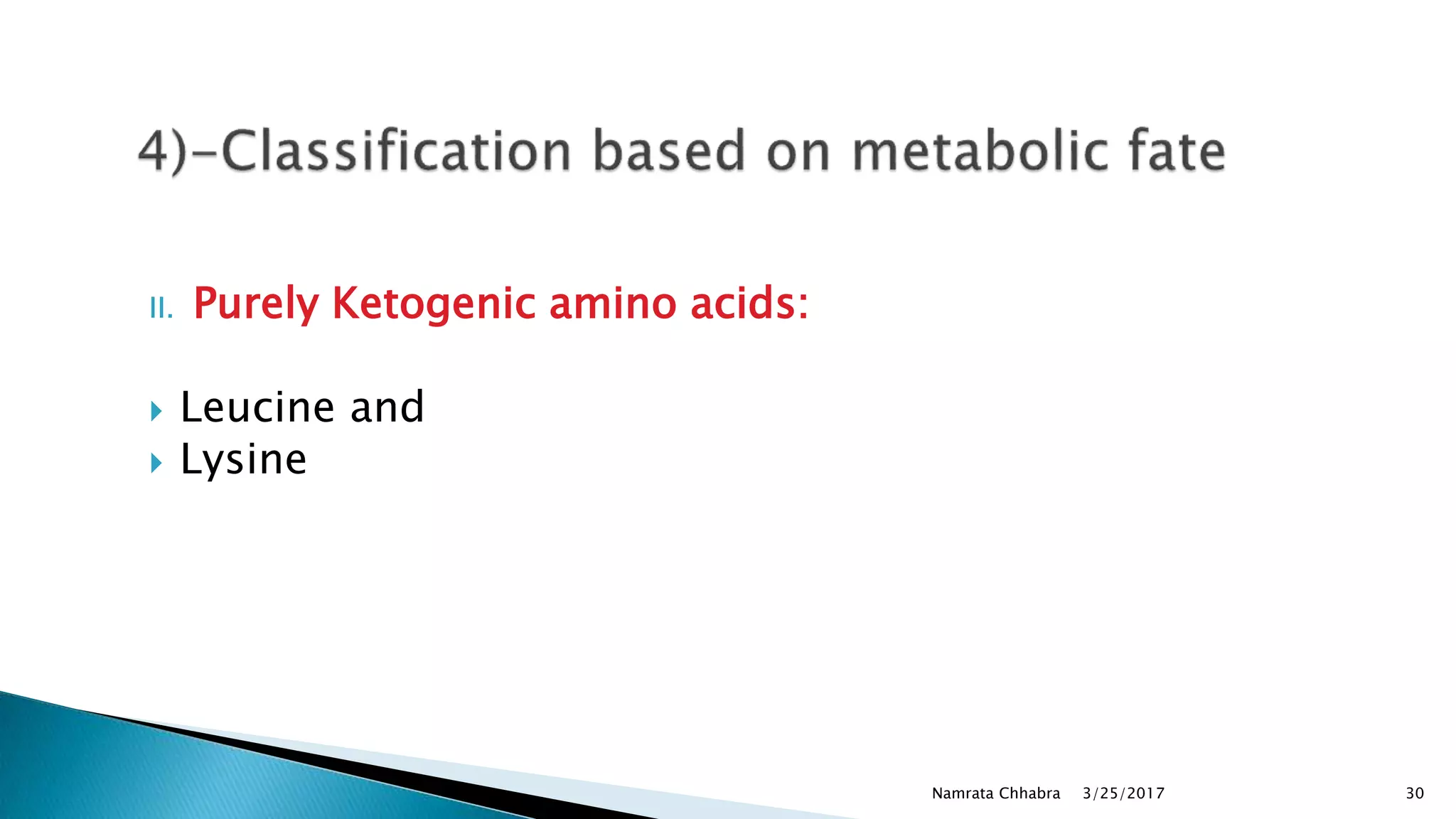
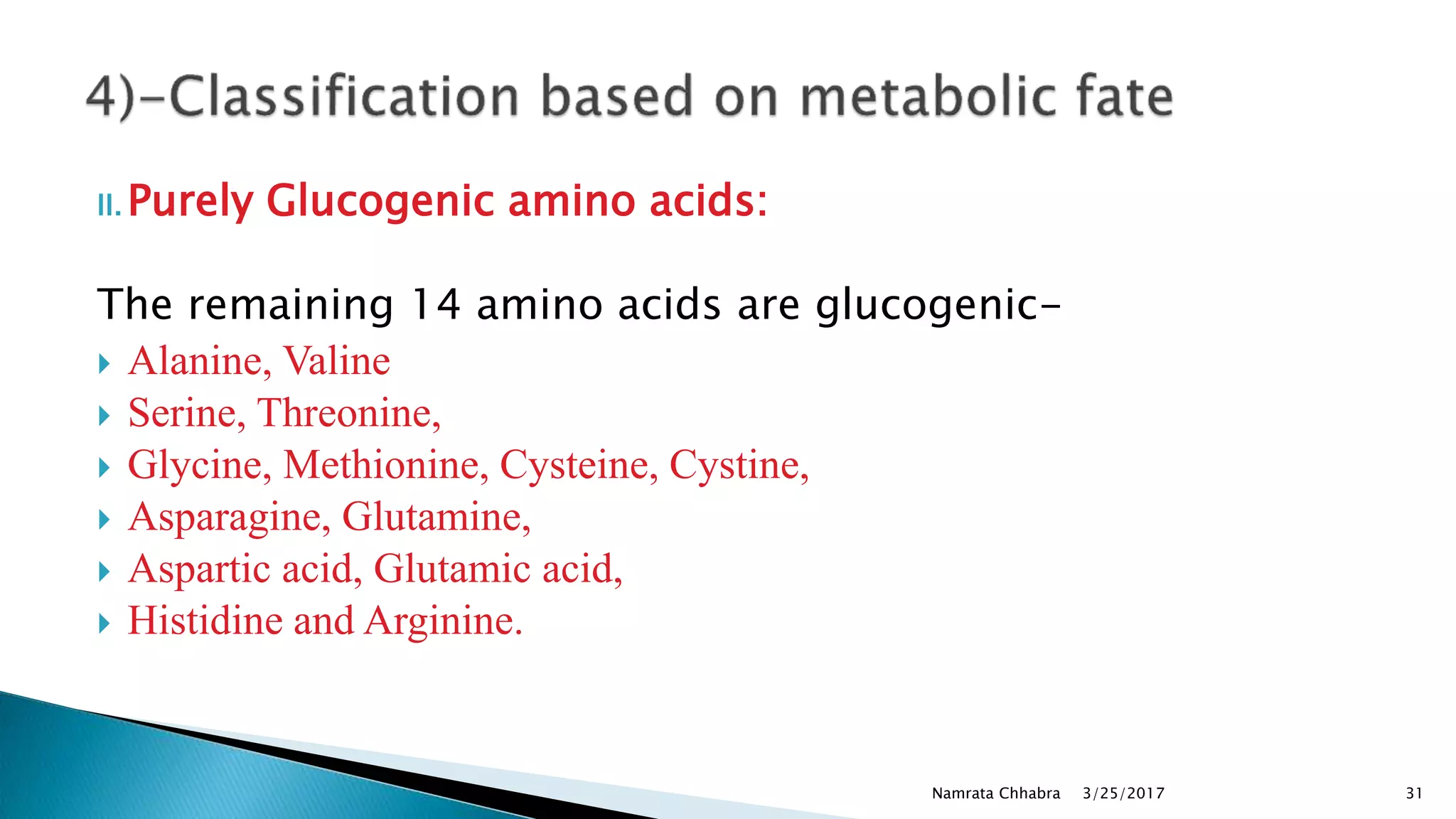
L-amino acids are the monomer units that make up the polypeptide chains of proteins. Short amino acid polymers called peptides also play important roles as hormones, hormone-releasing factors, neuromodulators, or neurotransmitters. There are 20 standard amino acids that are the basic building blocks of proteins in the human body. Amino acids can be classified in different ways, including by their structure, side chain properties, nutritional requirements, and metabolic fate.