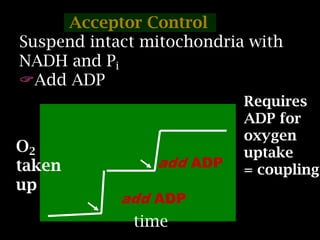
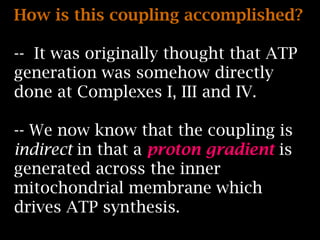
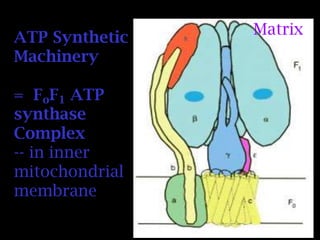
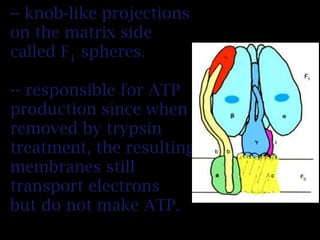
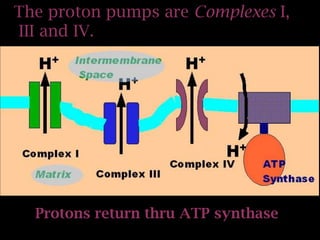
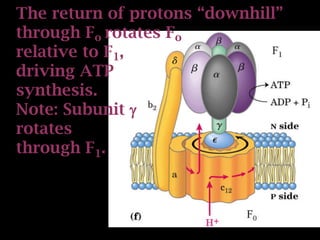
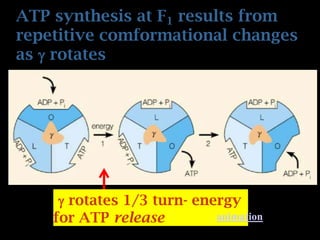
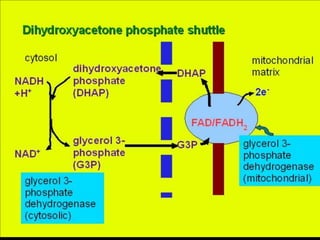
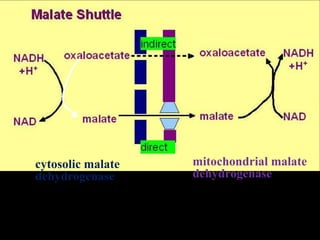
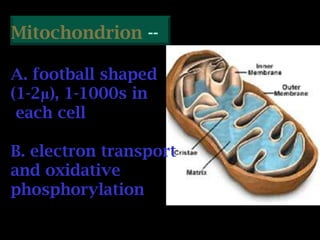
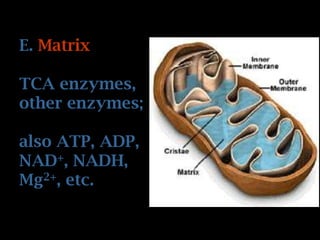
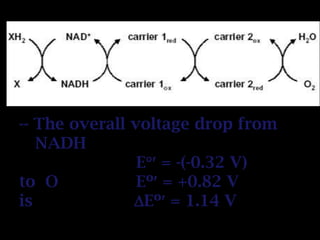
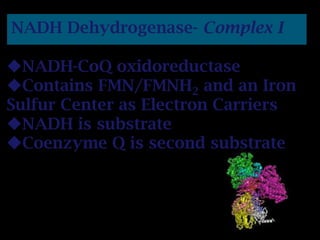
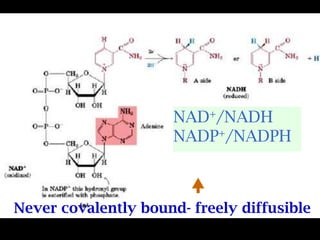
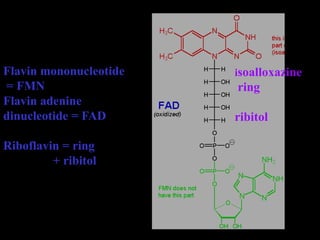
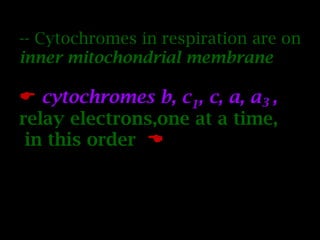
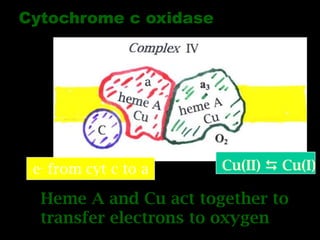
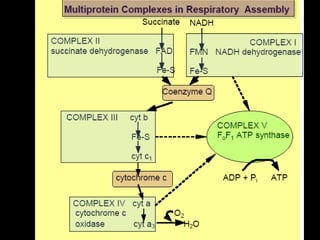
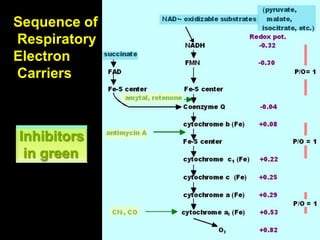
The document summarizes electron transport and oxidative phosphorylation. It describes how electrons from NADH and FADH2 are transported via carriers in the mitochondrial electron transport system to oxygen, with energy released used to synthesize ATP. Protons are pumped from the mitochondrial matrix to the intermembrane space, building a proton gradient that drives ATP synthesis by ATP synthase as protons flow back into the matrix. This chemiosmotic coupling allows efficient conversion of electron potential energy to chemical energy in the form of ATP.








![What about energy and ATP
stoichiometry? -- measured
-- 220 kJ/mole from NADH oxidation
-- Each ATP produced: ADP + Pi ATP
G = +30.5 kJ/mole
[3 (30.5)/220] 100 = 41% efficiency](https://image.slidesharecdn.com/etsslides-140330135302-phpapp01/85/ELECTRON-TRANSPORT-AND-OXIDATIVE-PHOSPHORYLATION-24-320.jpg)