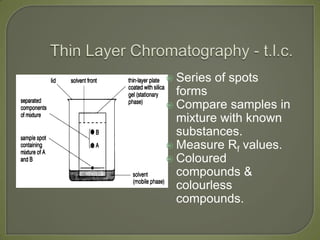
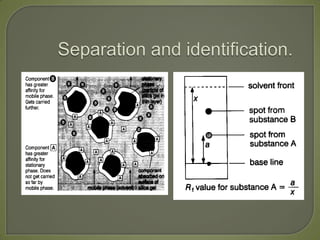
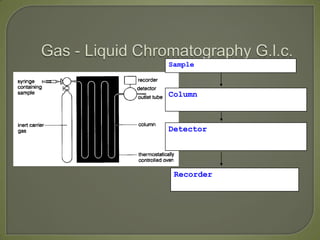
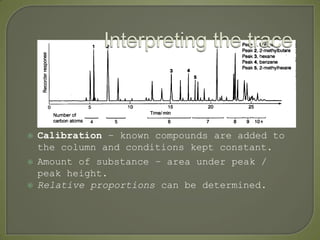
Chromatography is a technique used to separate and identify components of mixtures using the principle of partition between two phases, a stationary phase and a mobile phase. There are several types including paper, thin layer, and gas-liquid chromatography. Gas-liquid chromatography involves passing a sample through a column containing a stationary liquid phase using an inert gas as the mobile phase, which separates the components into individual peaks that can be analyzed to identify substances.