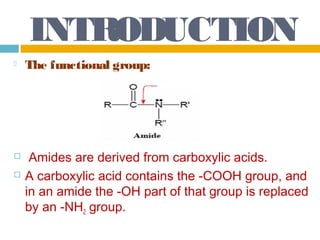
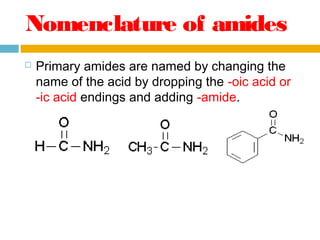
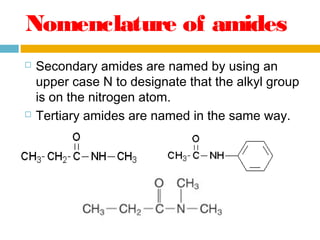
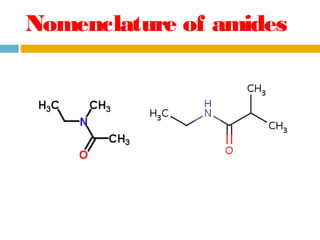
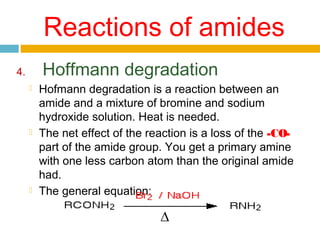
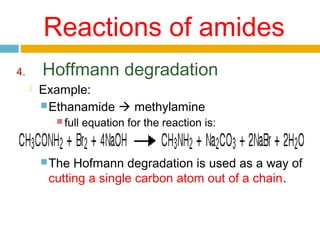
1. Amides are derived from carboxylic acids by replacing the -OH group of the carboxylic acid with an -NH2 group. Primary amides are named by changing the acid name to the acid name amide. Secondary and tertiary amides use uppercase N to designate the alkyl group on nitrogen.
2. Amides can be prepared from carboxylic acids or acid chlorides. From acids, an ammonium salt is formed which dehydrates to the amide upon heating. From acid chlorides, the acid chloride reacts with ammonia.
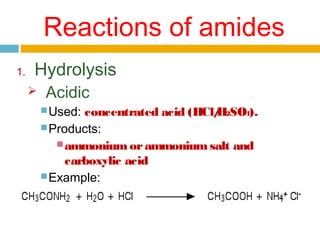
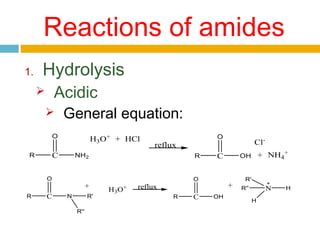
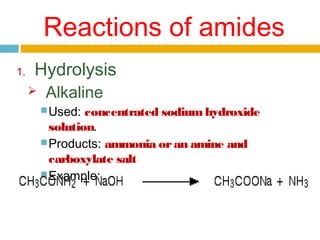
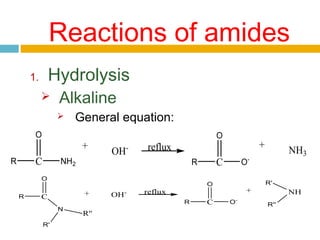
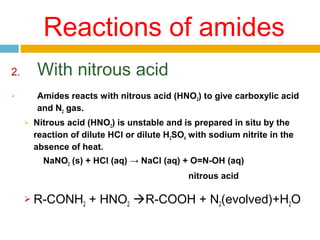
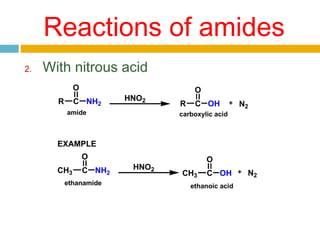
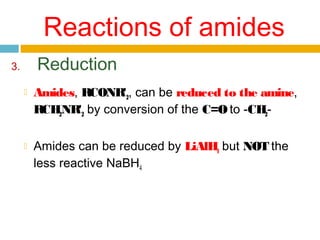
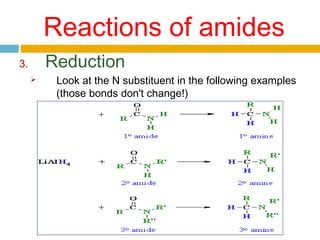
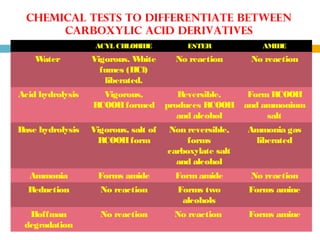
3. Amides undergo hydrolysis to form carboxylic acids and ammonia/ammonium salts. They also react with nitro