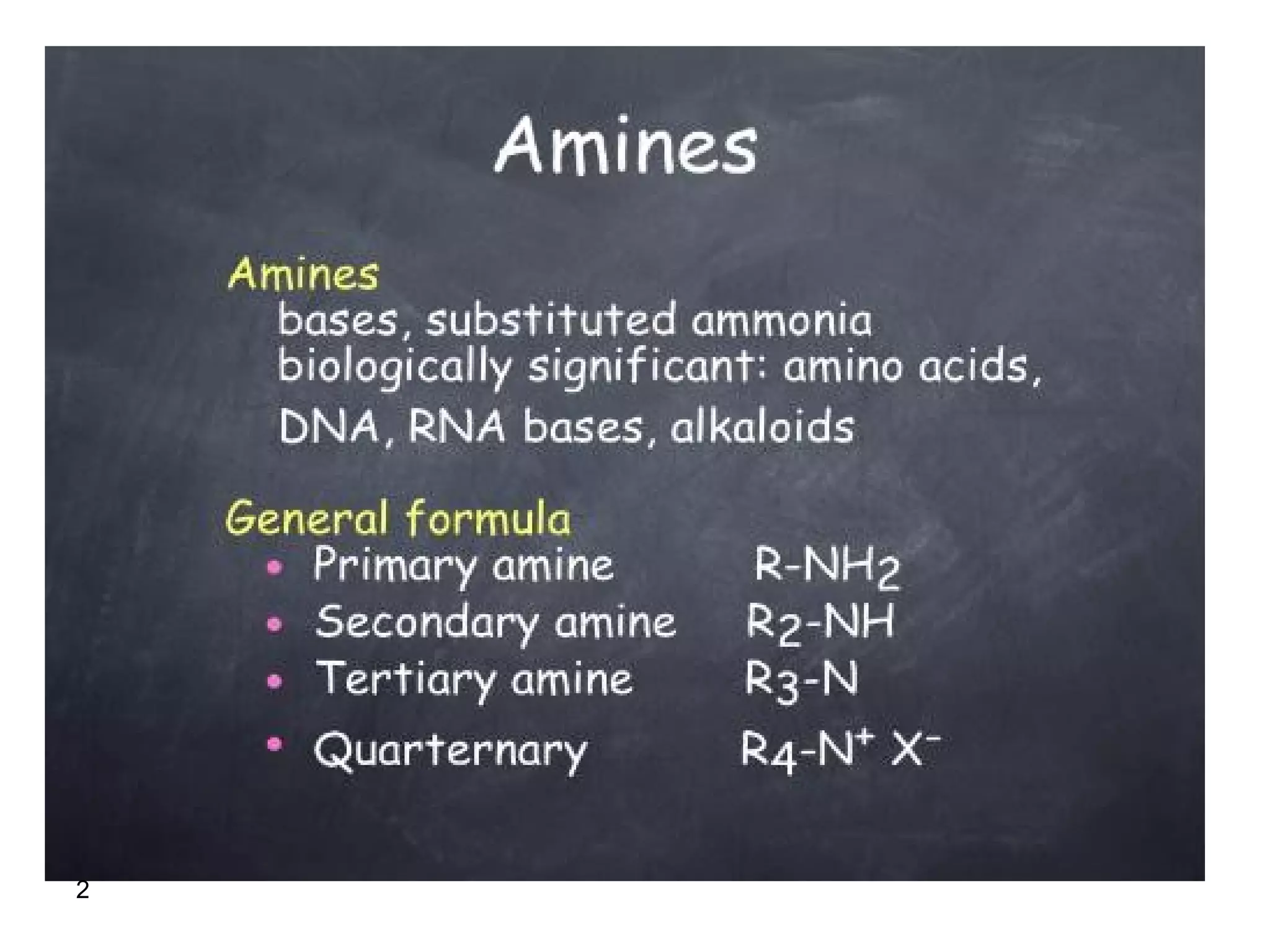
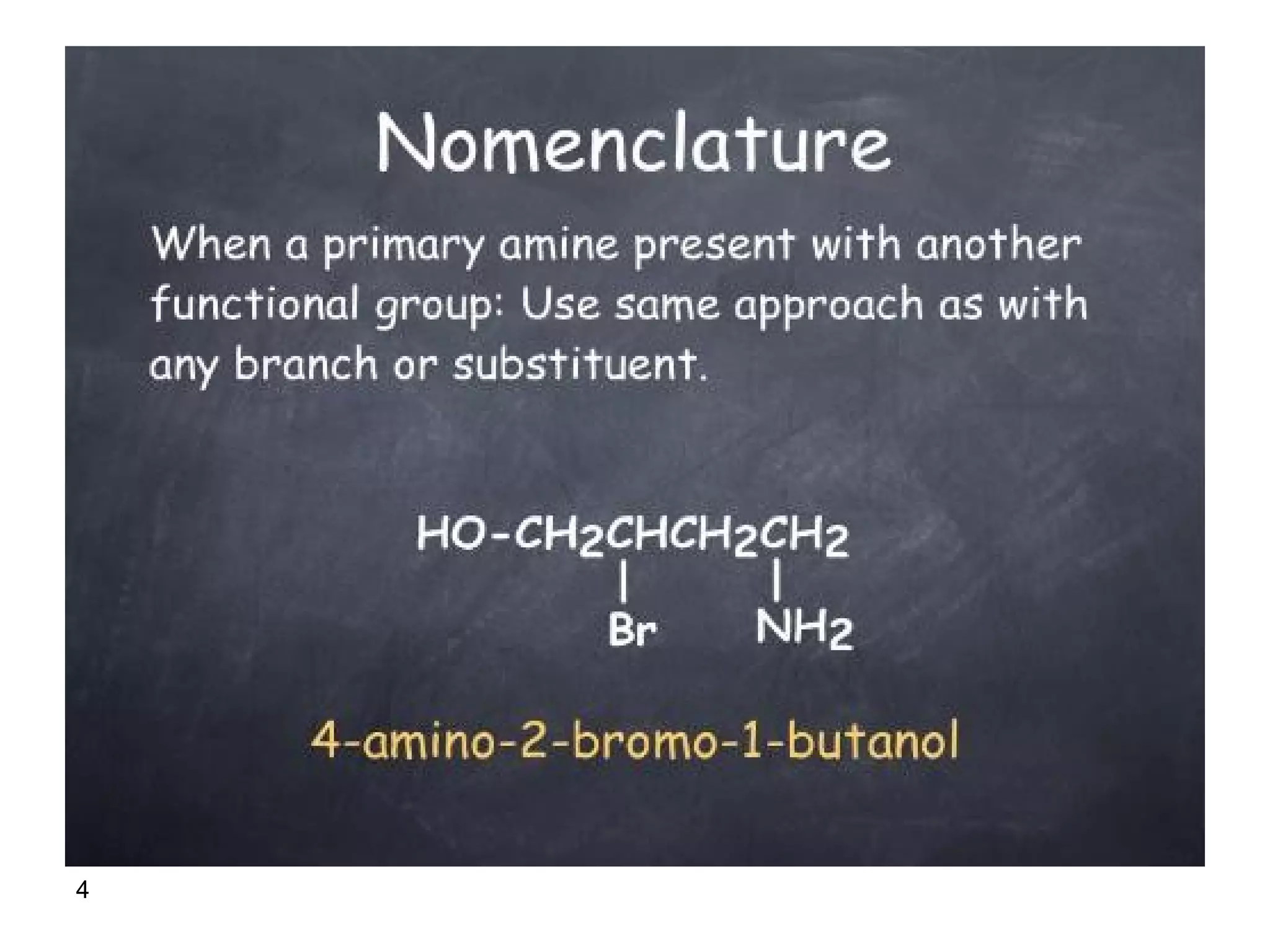
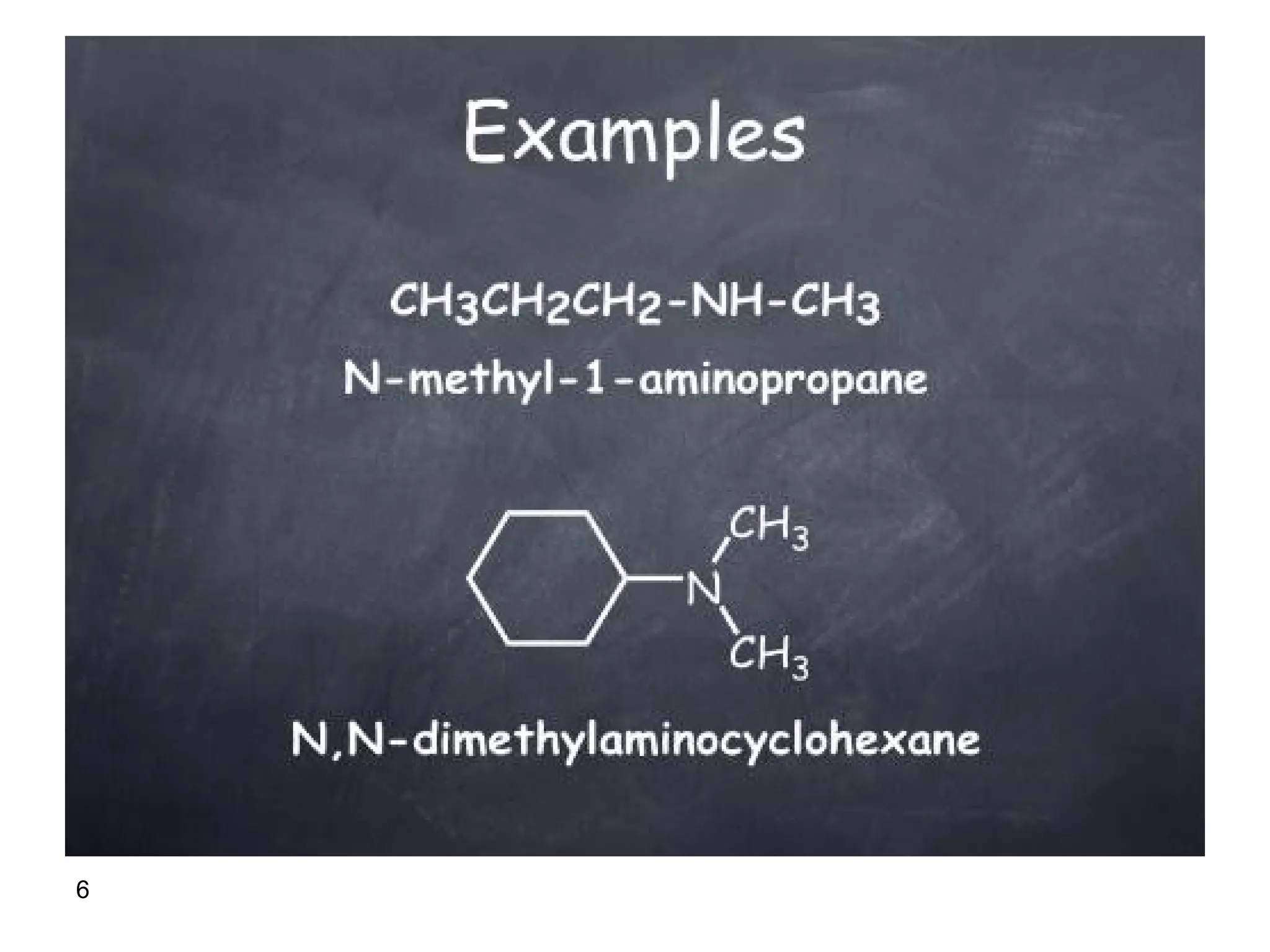
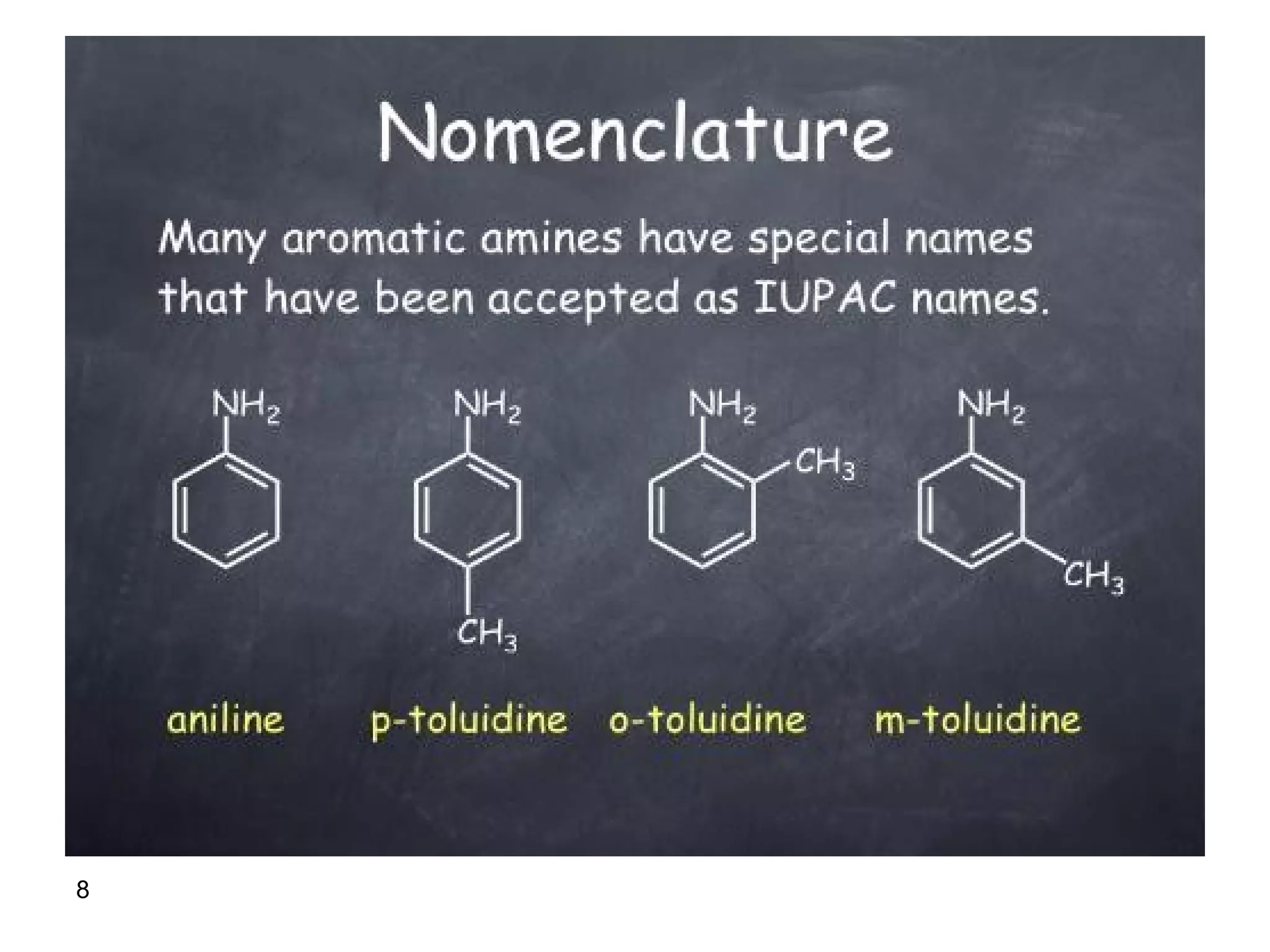
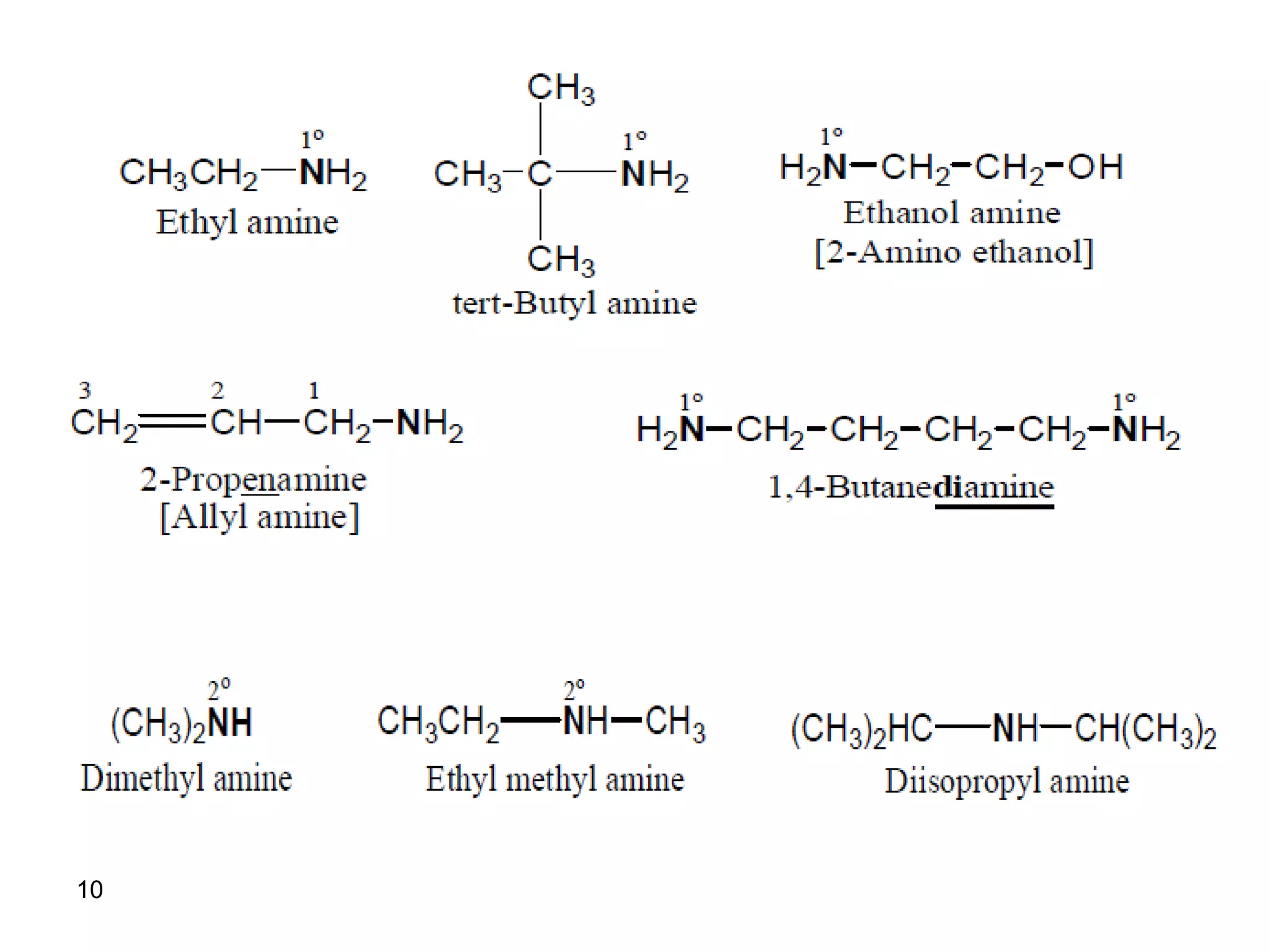
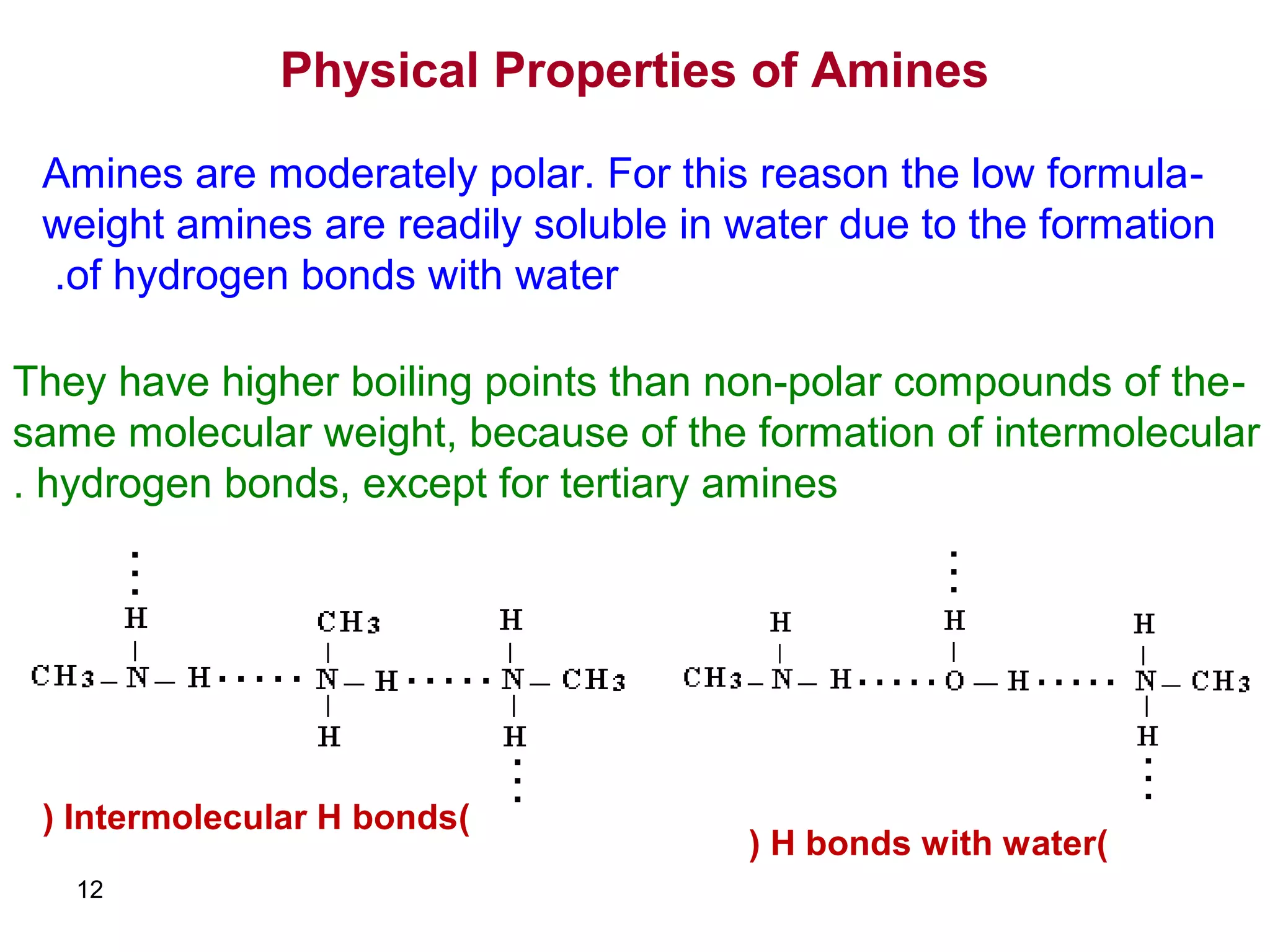
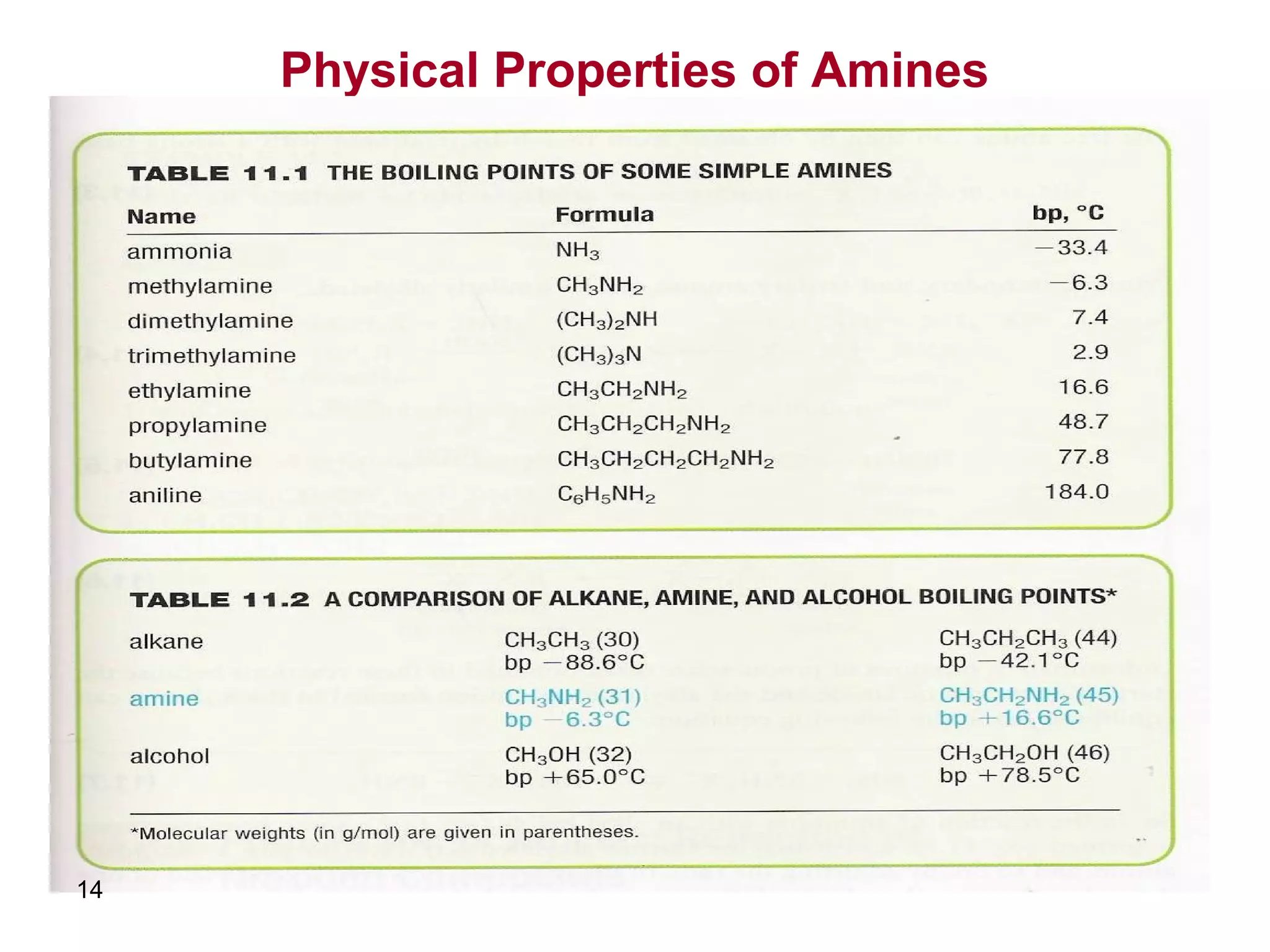
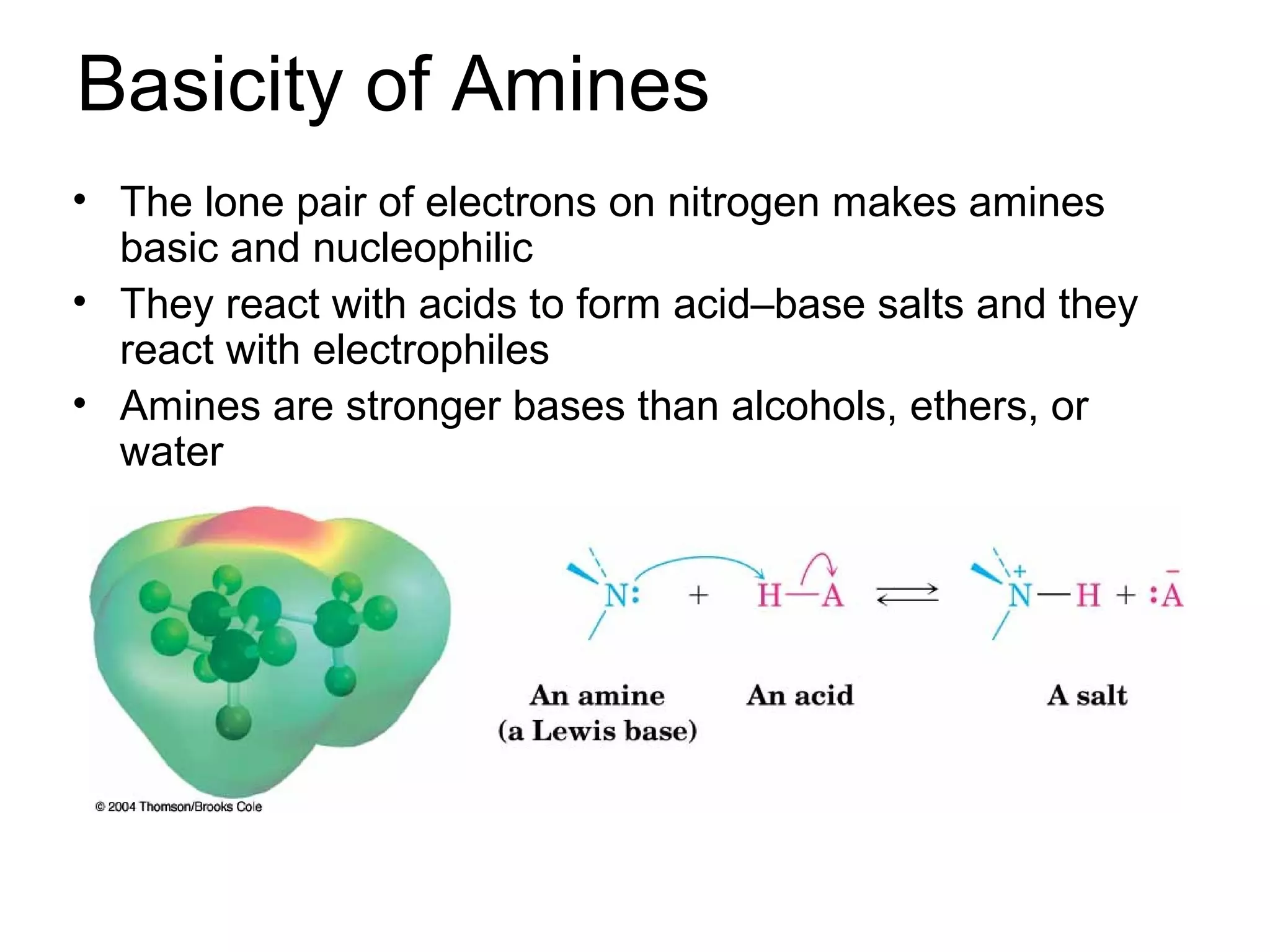
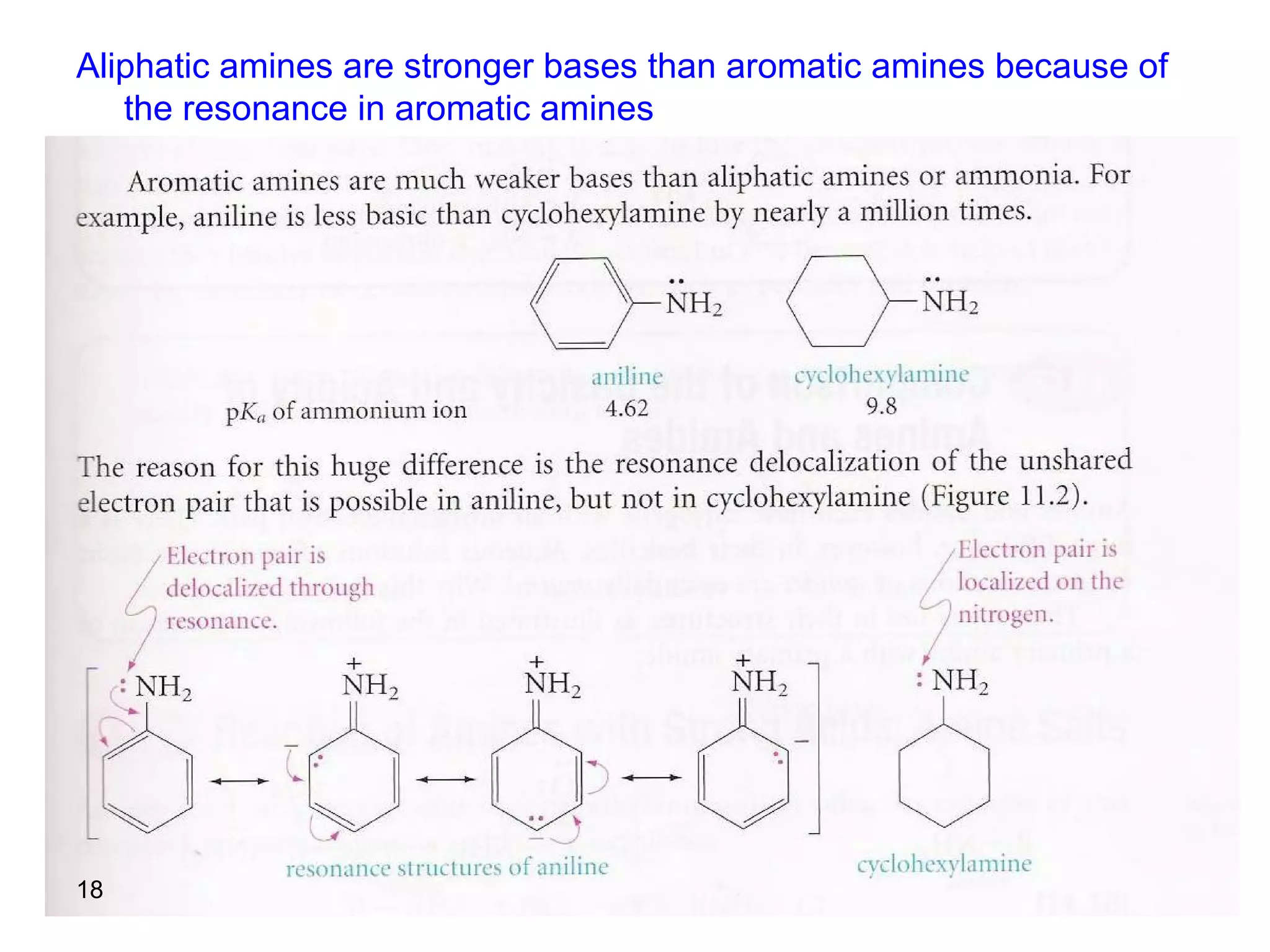
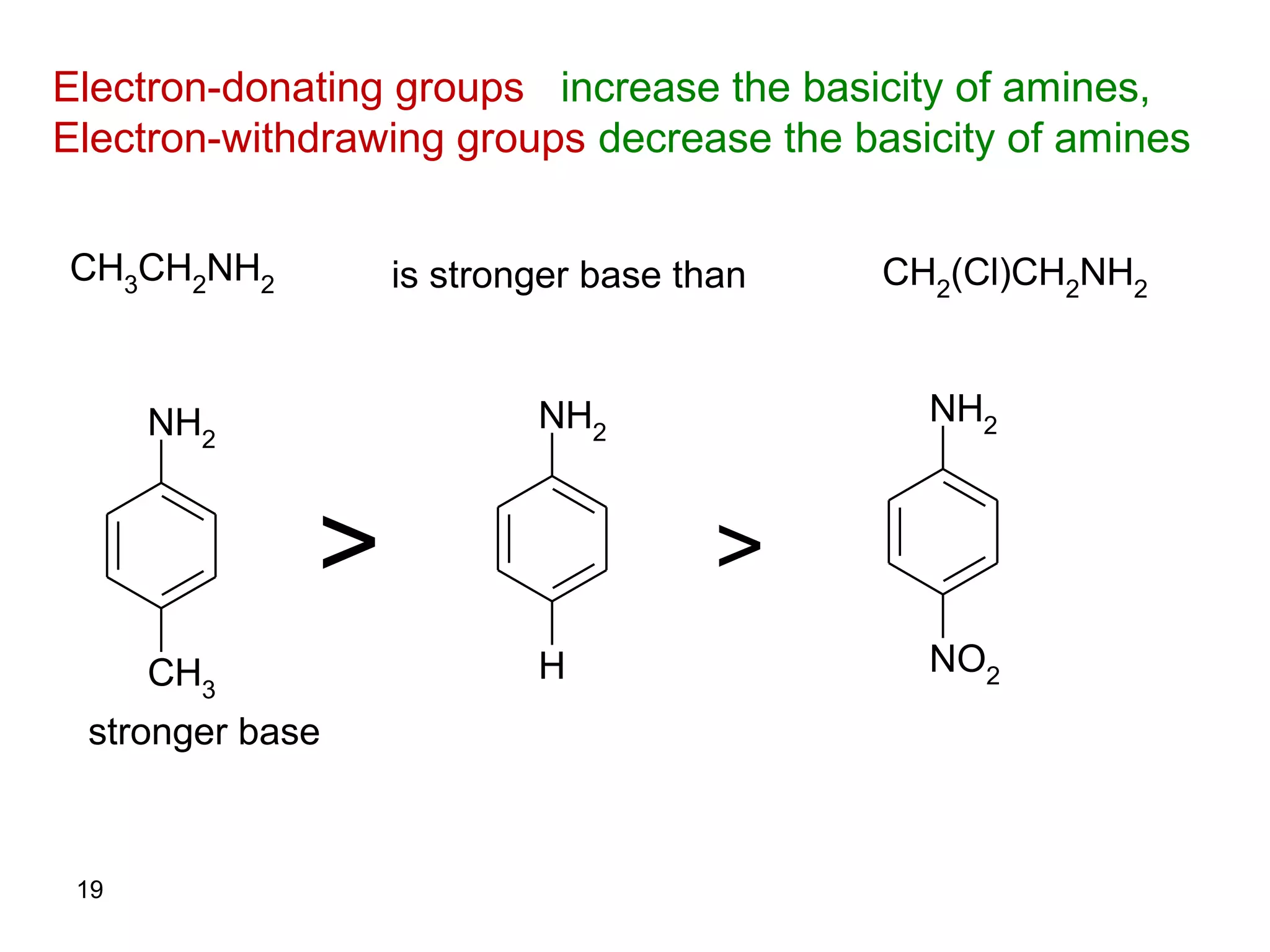
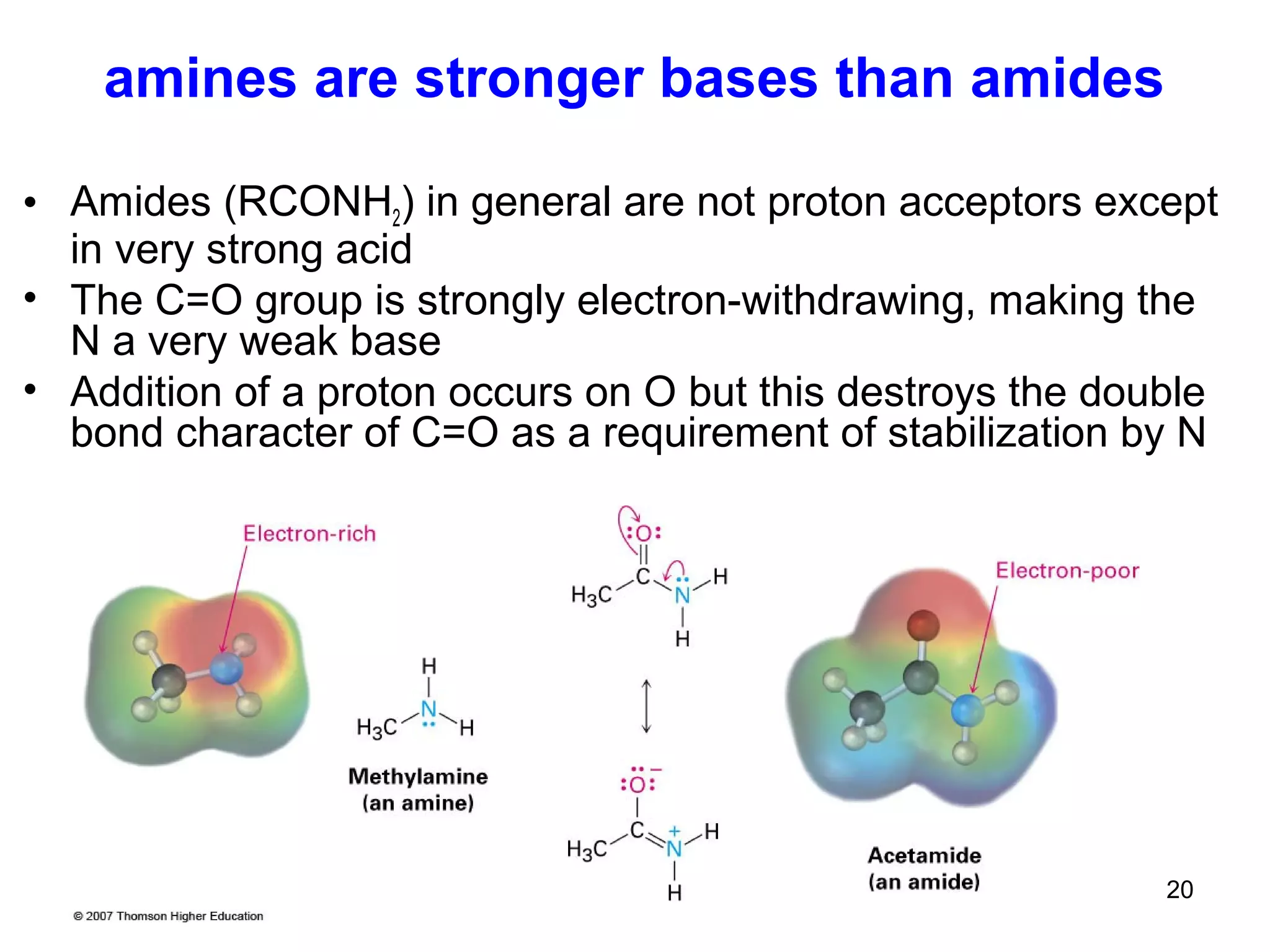
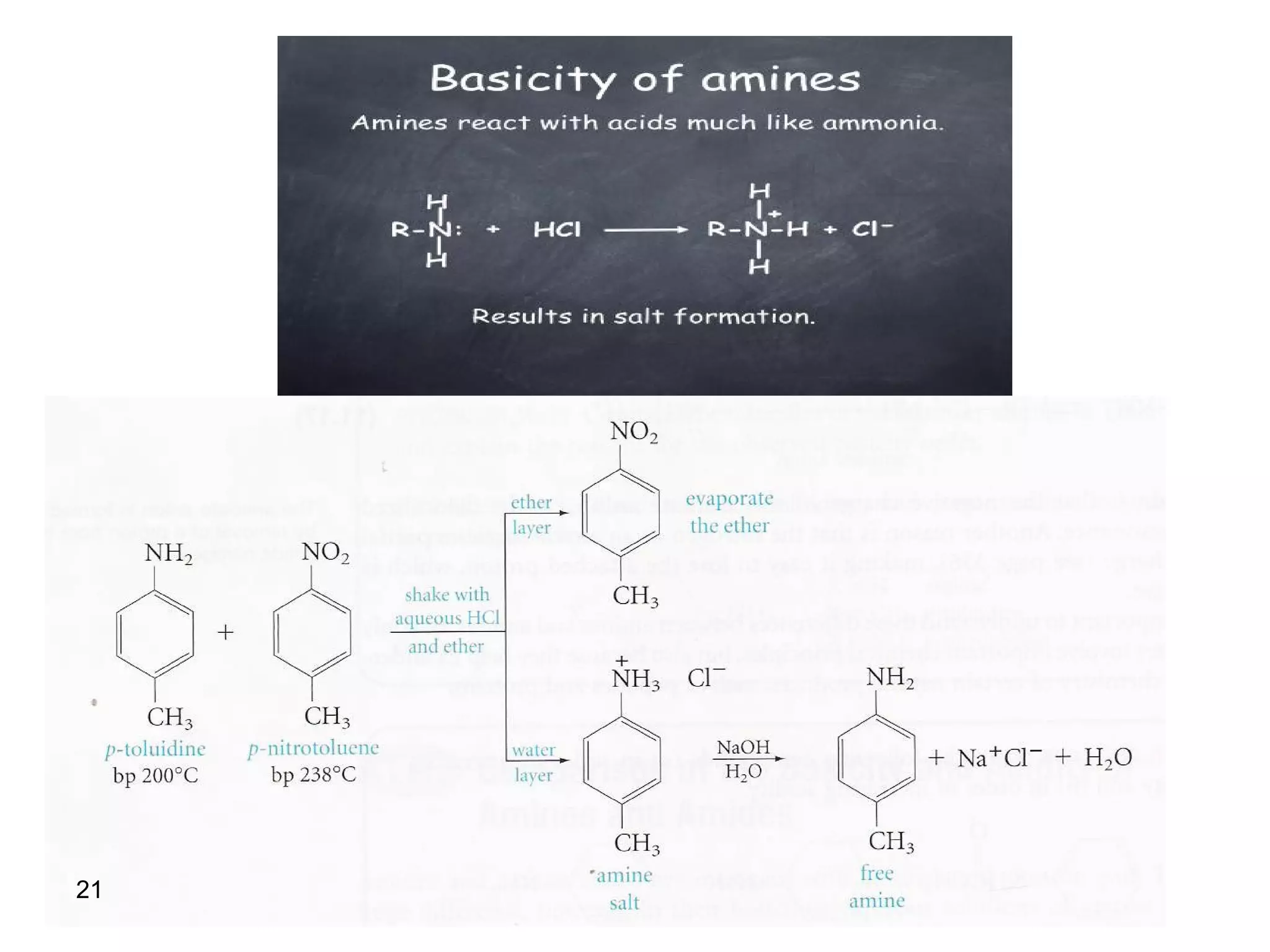
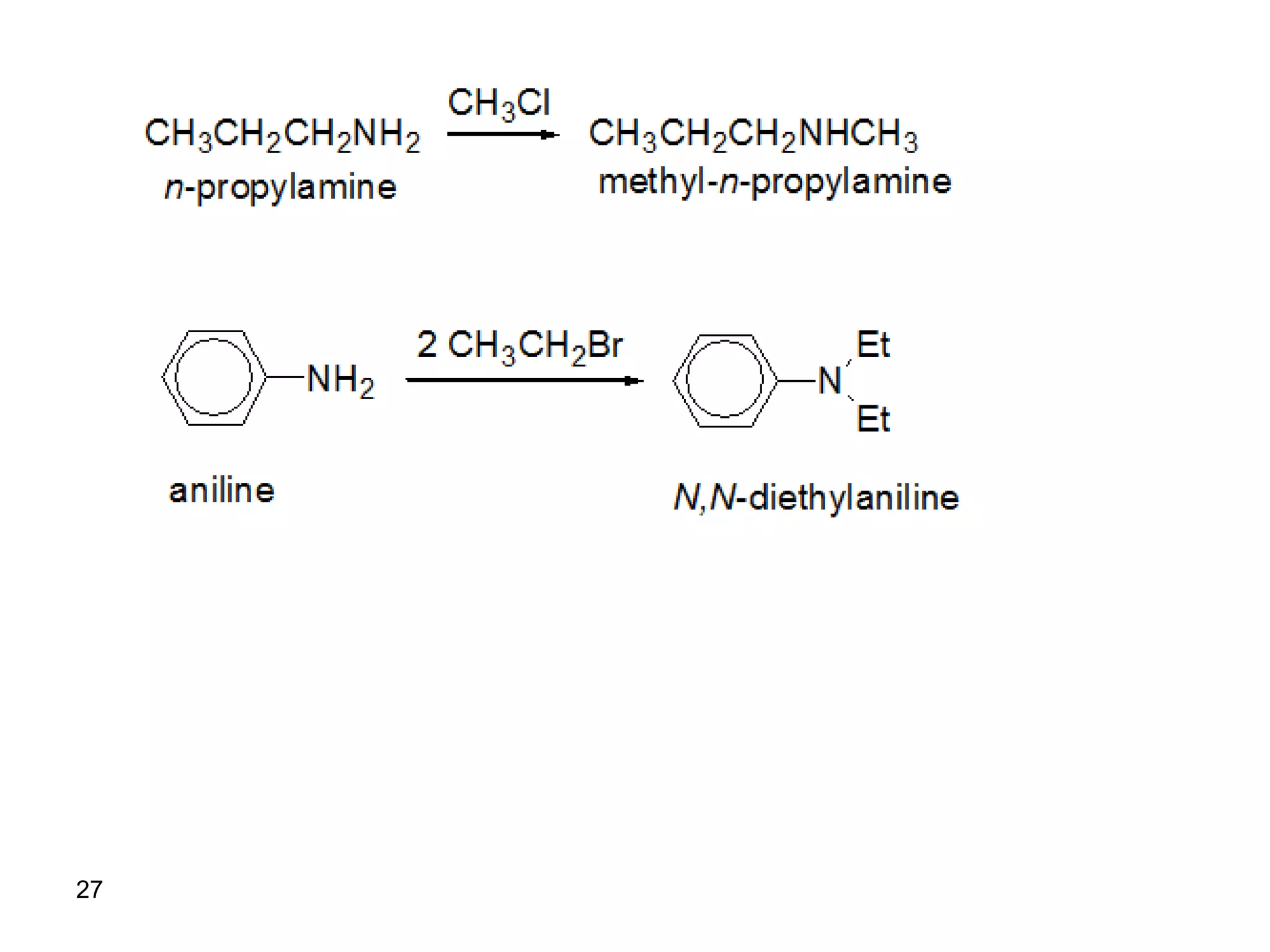
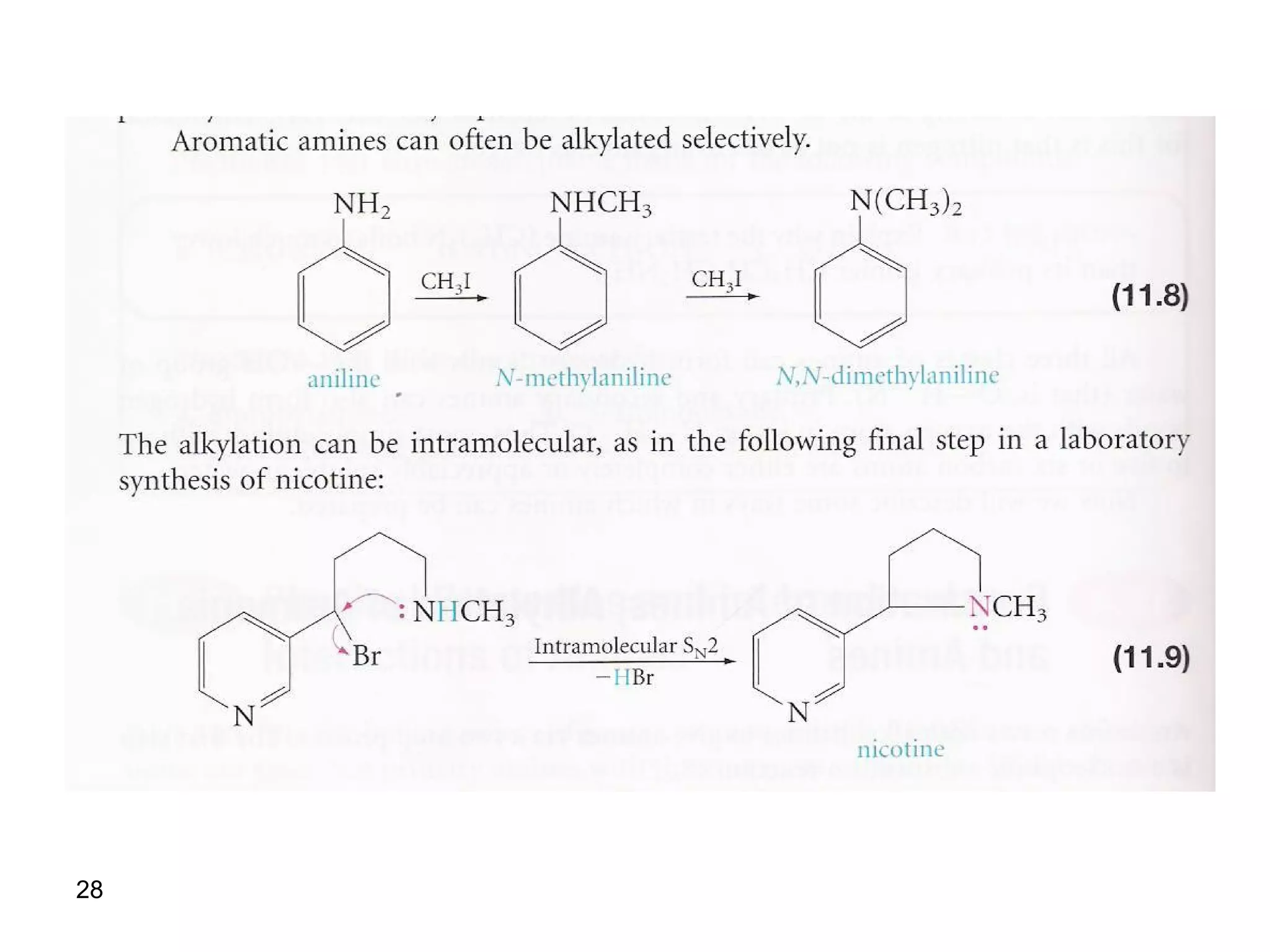
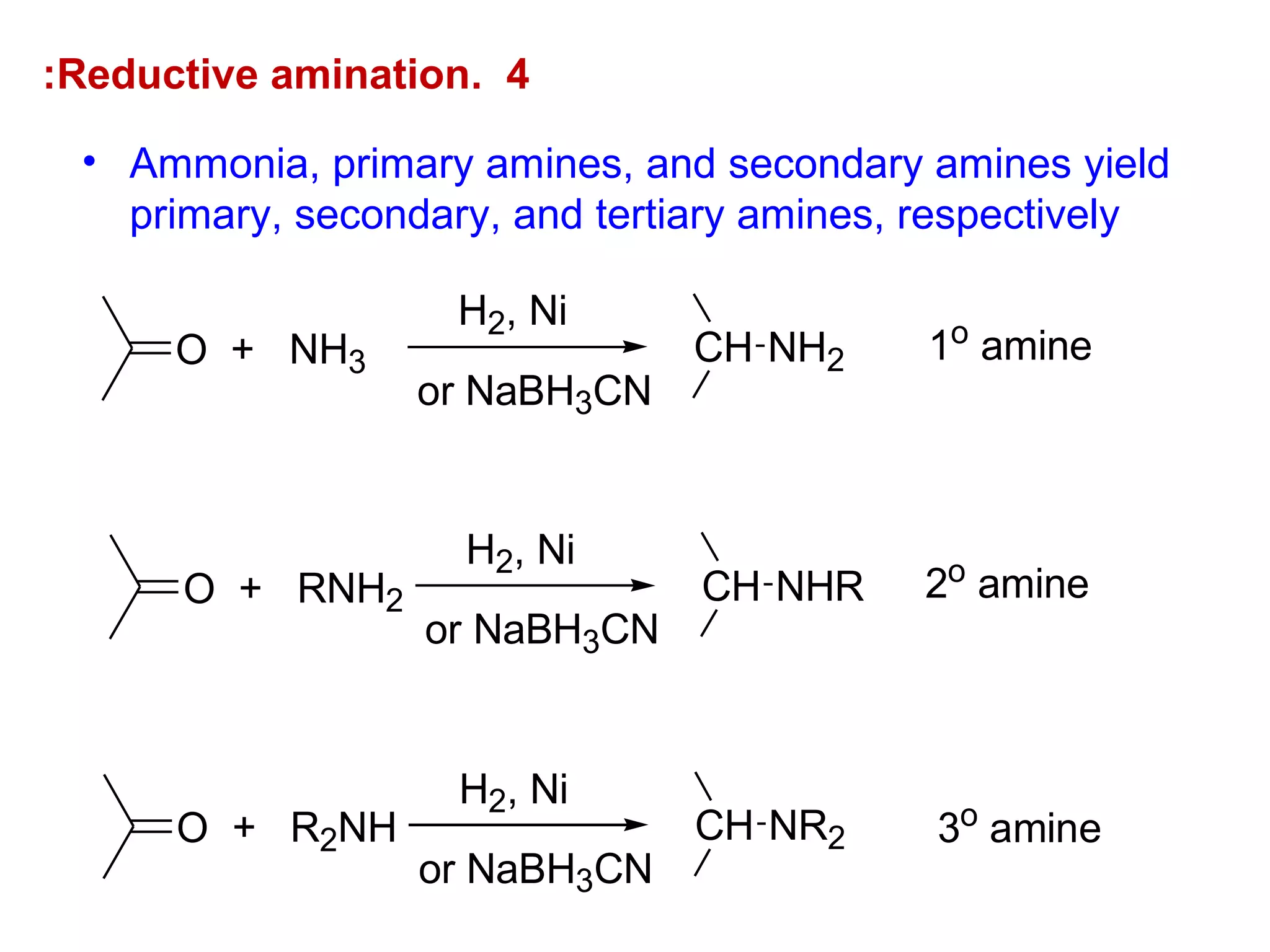
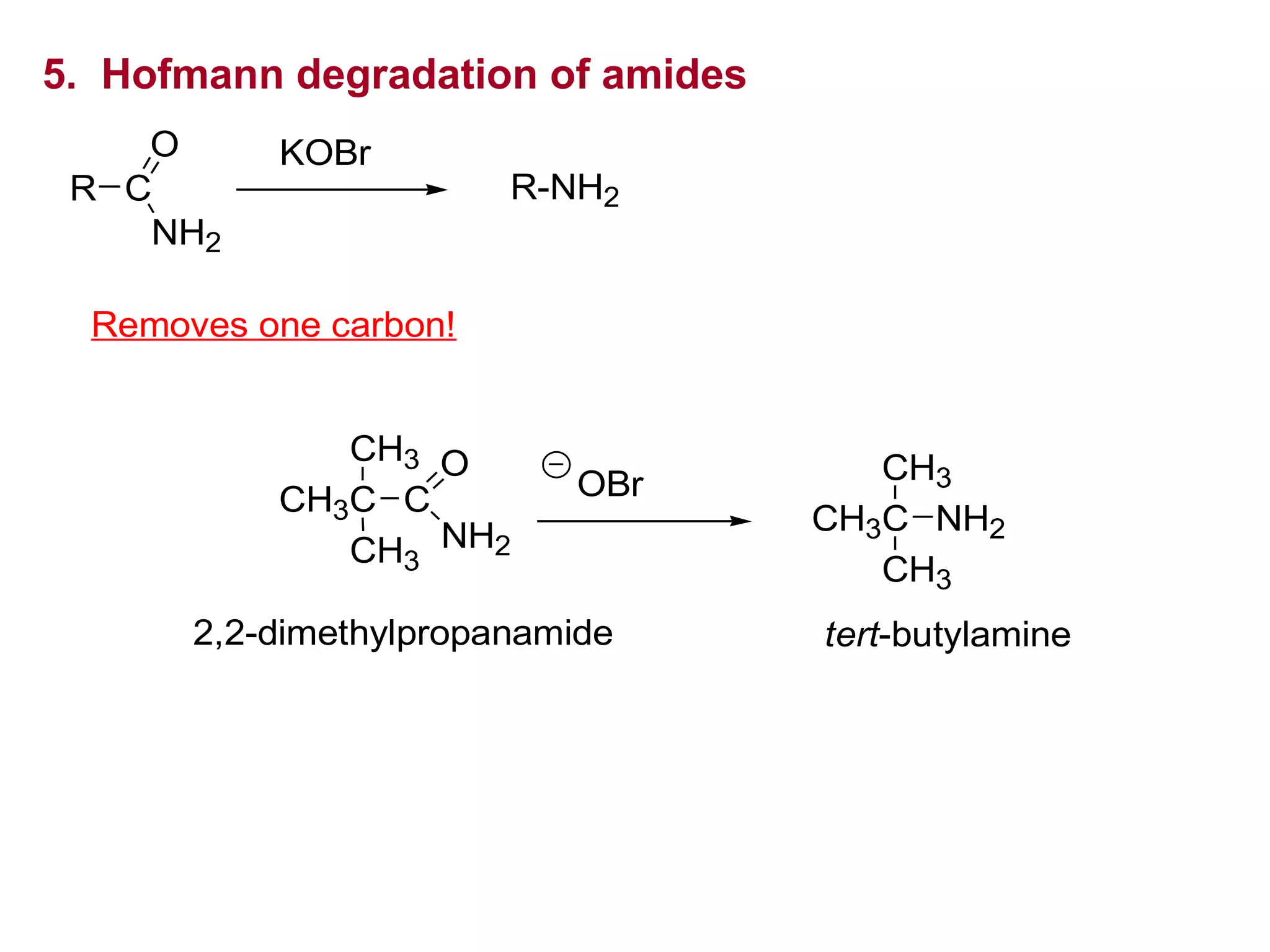
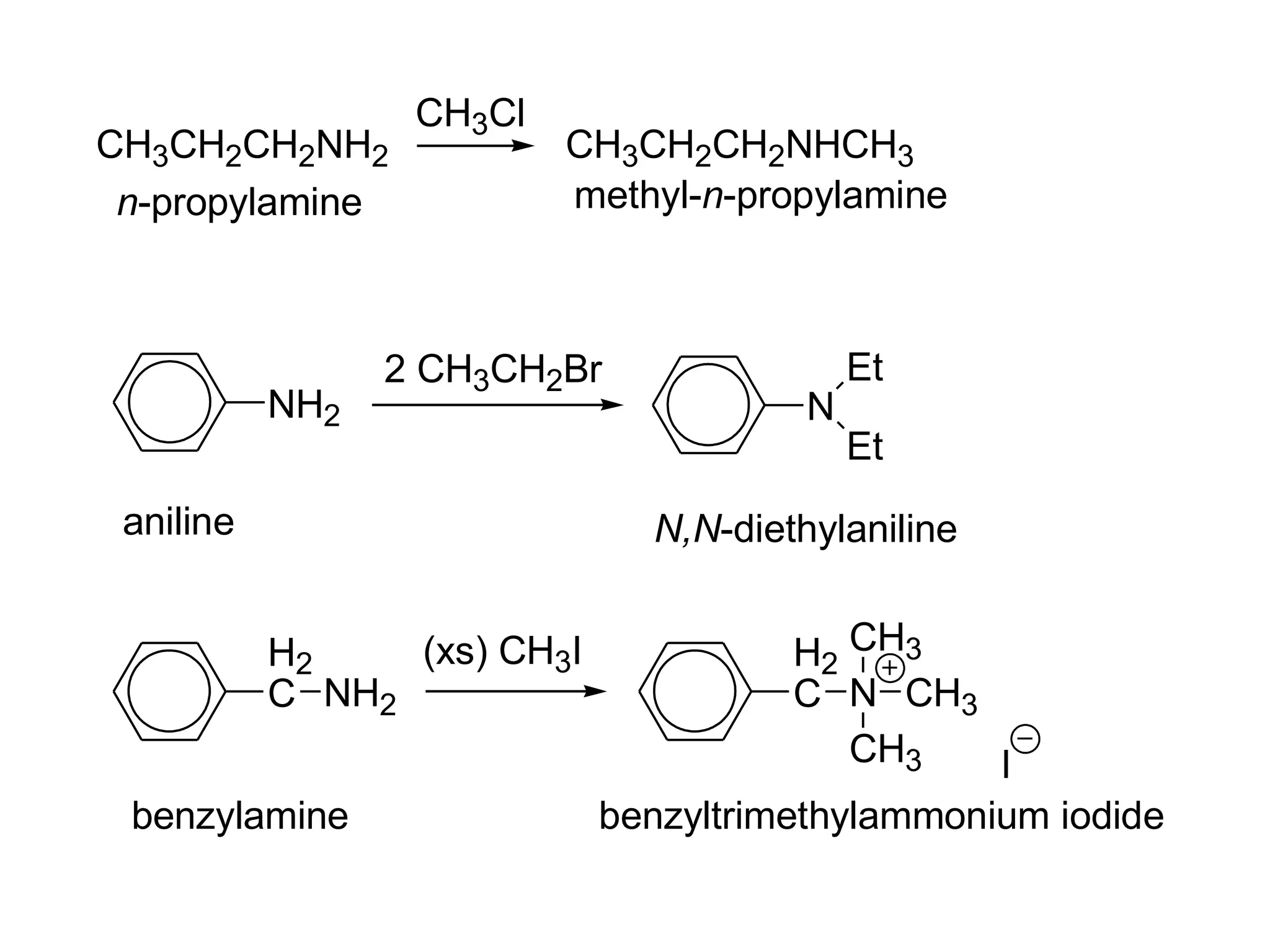
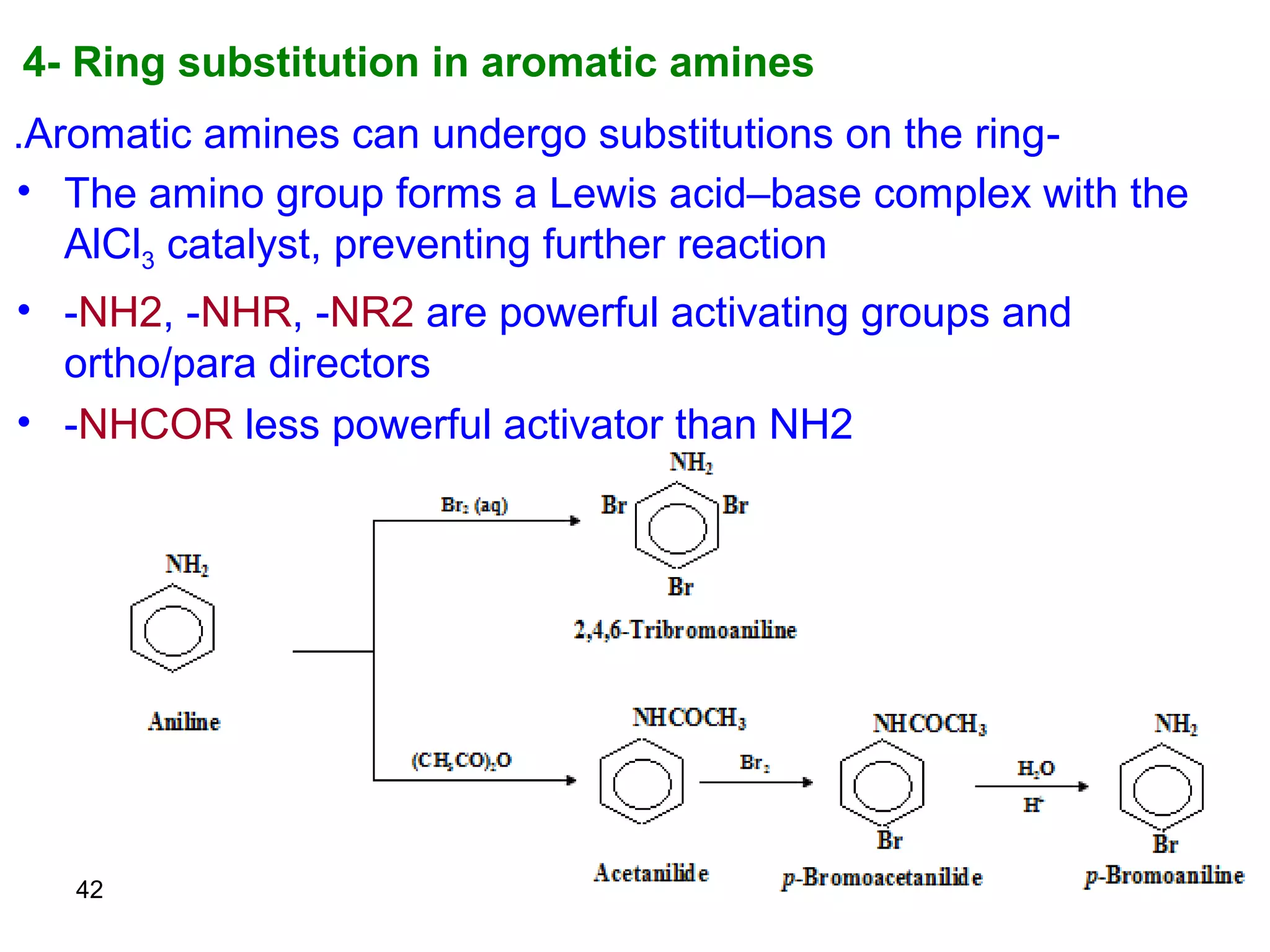
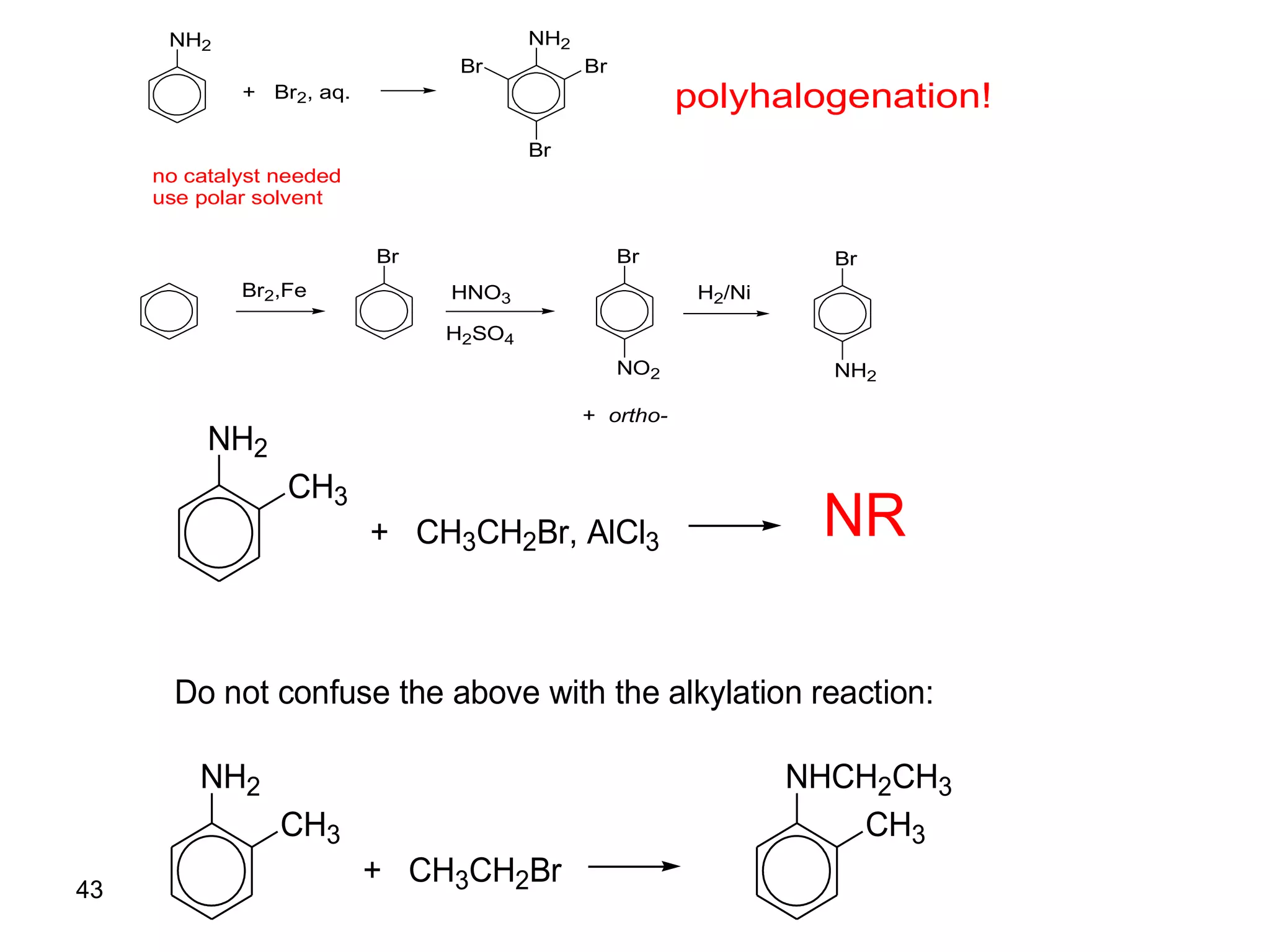
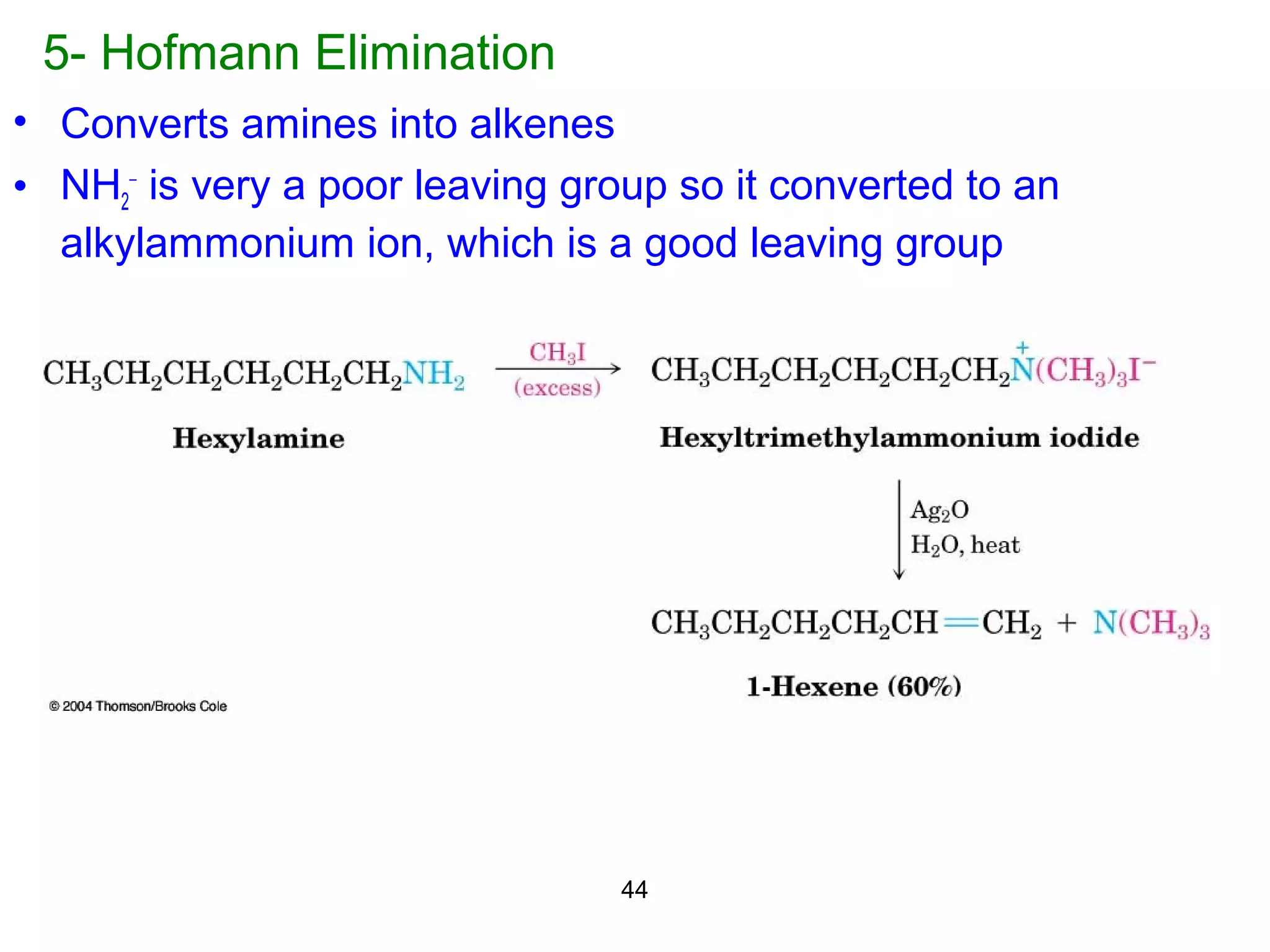
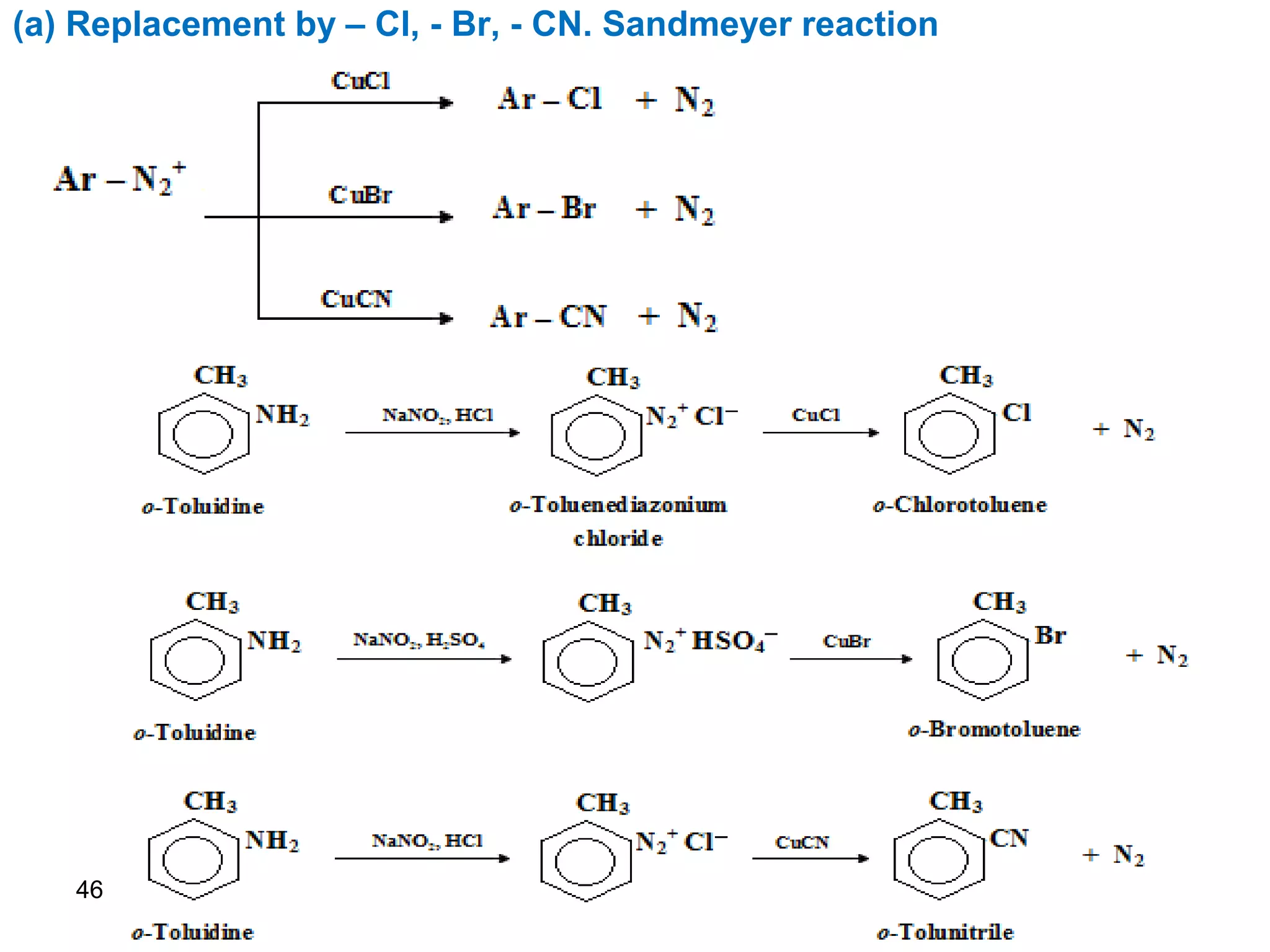
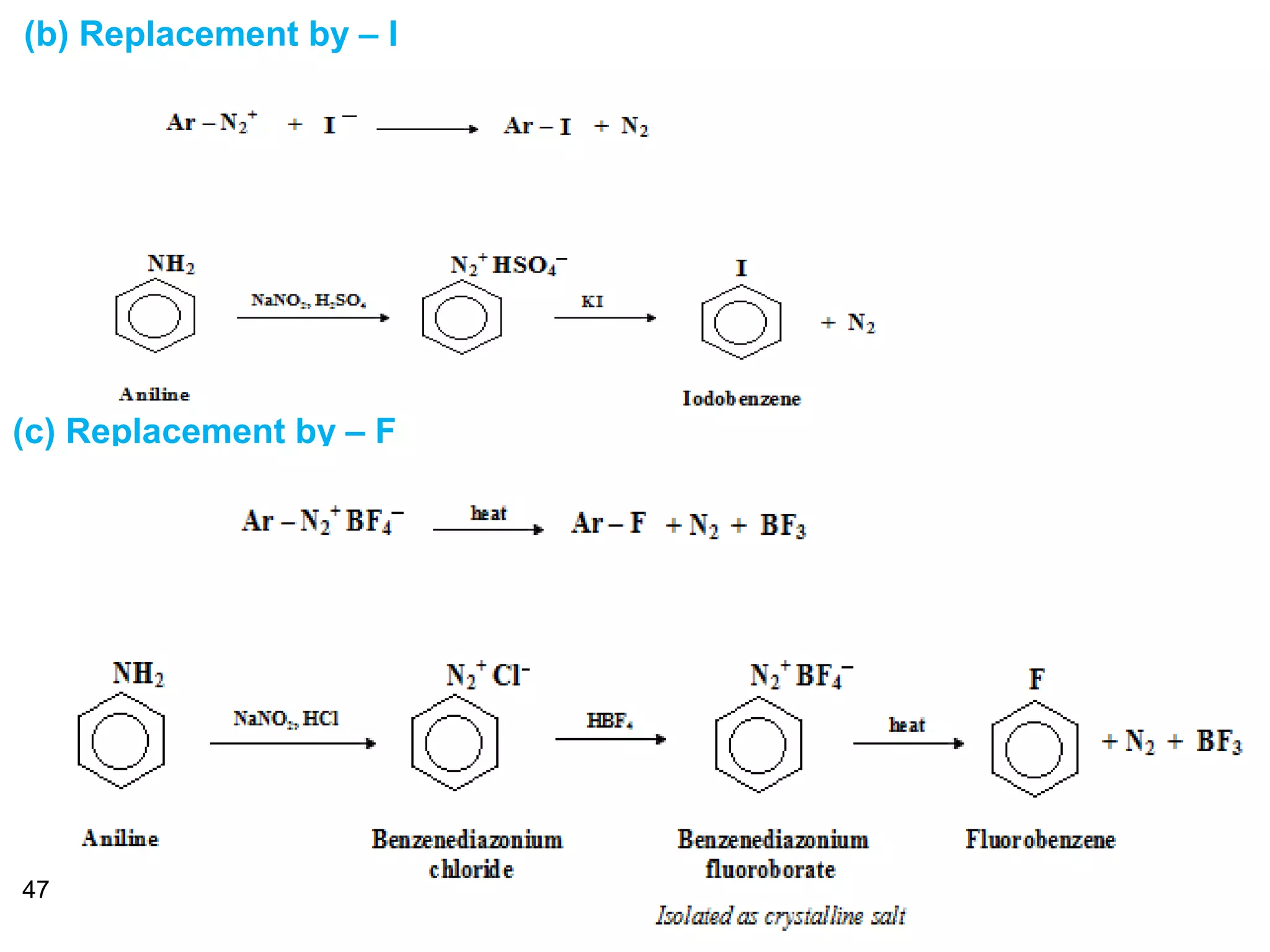
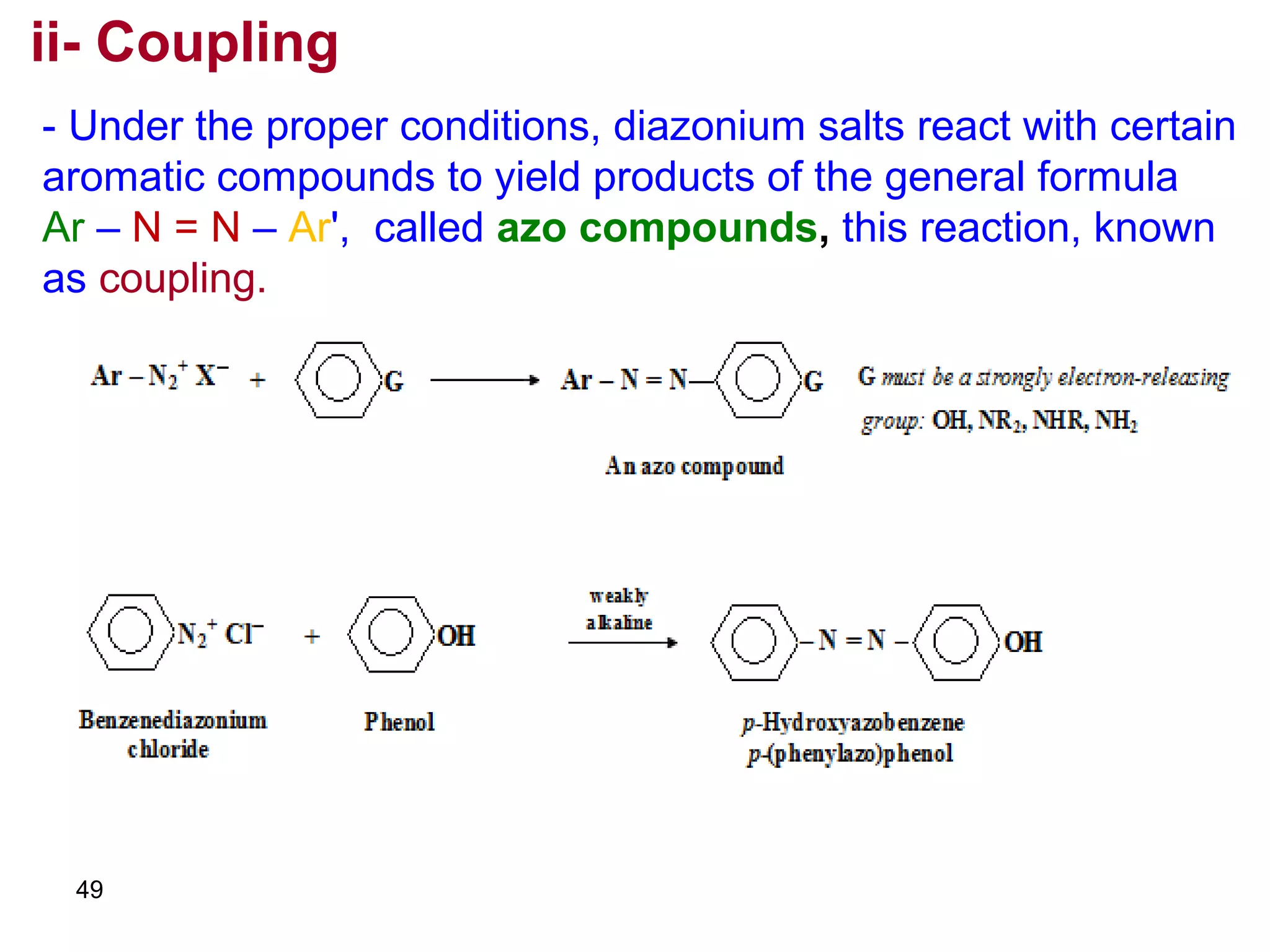
This document discusses the properties and reactions of amines. It describes how amines are moderately polar and soluble in water due to hydrogen bonding. Their boiling points are higher than non-polar compounds due to intermolecular hydrogen bonding. Amines are basic due to the lone pair of electrons on nitrogen. Common reactions of amines include salt formation, alkylation, conversion to amides, aromatic substitution, Hofmann elimination, and formation of diazonium salts. Diazonium salts can undergo replacement or coupling reactions.