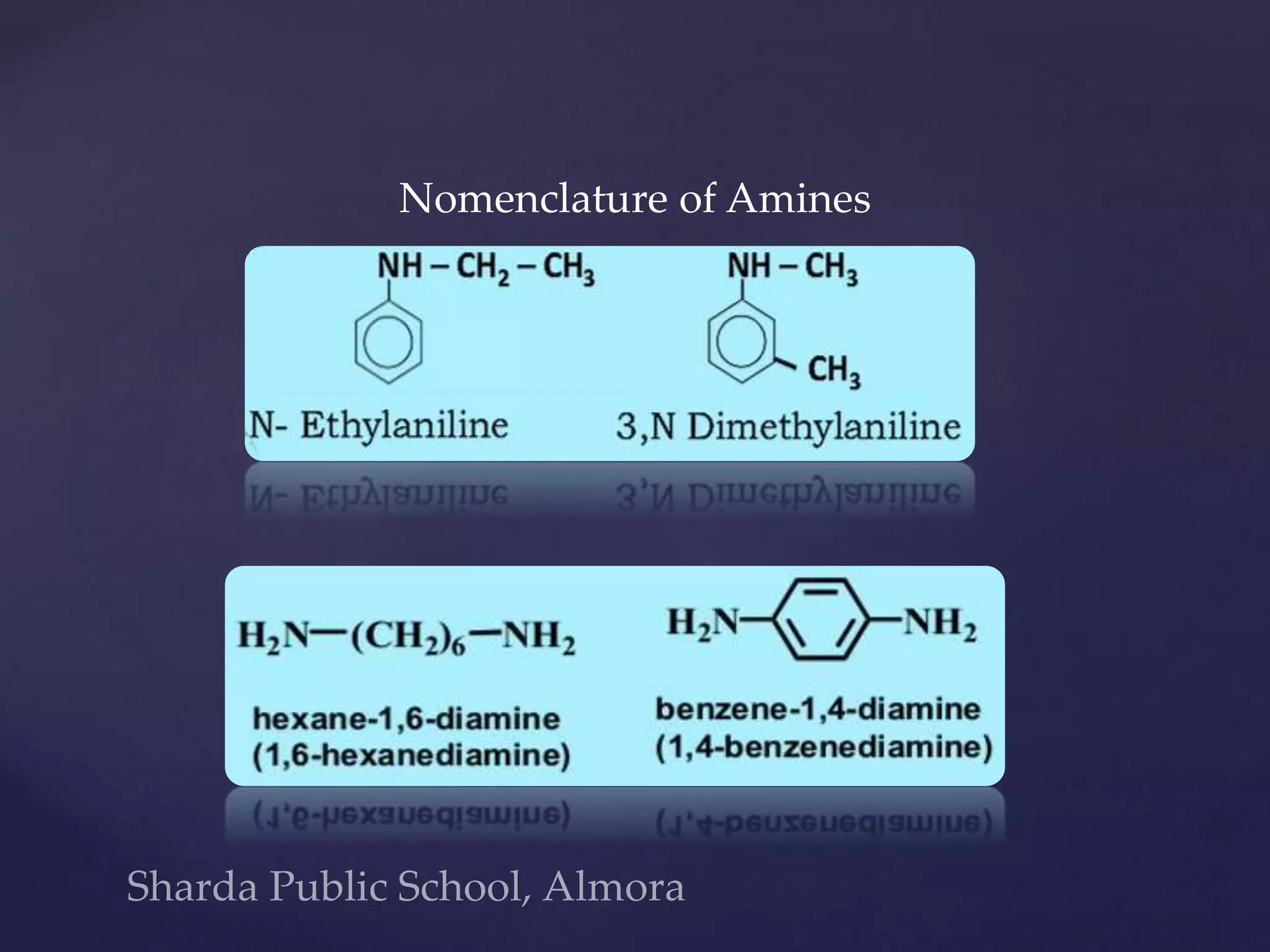
This document discusses amines, which are organic compounds derived from ammonia where an alkyl, cycloalkyl, or aryl group is bonded to the nitrogen atom. Amines are classified as primary, secondary, or tertiary based on how many hydrocarbon groups are bonded to the nitrogen. Primary amines have one hydrocarbon group and two hydrogens, secondary have two hydrocarbon groups and one hydrogen, and tertiary have three hydrocarbon groups. The document discusses the properties, reactions, preparation methods, and nomenclature of amines.