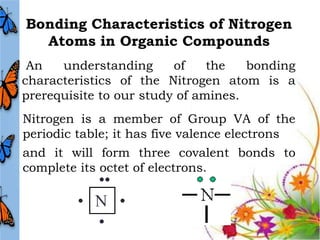
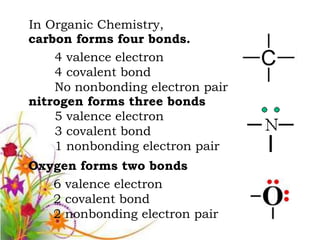
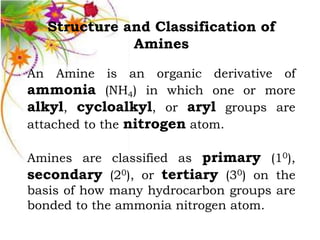
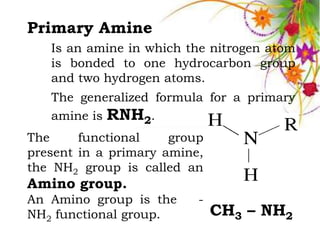
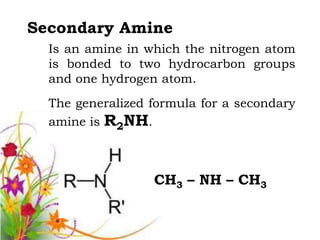
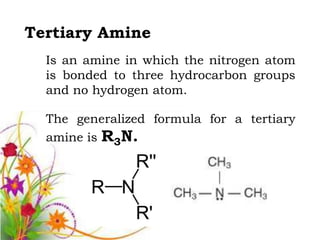
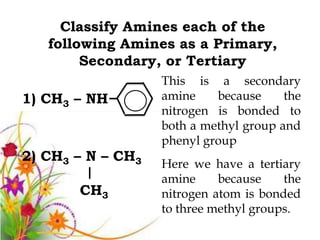
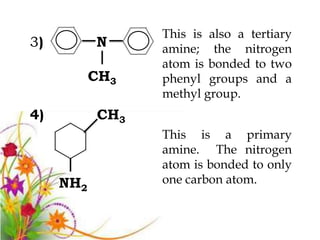
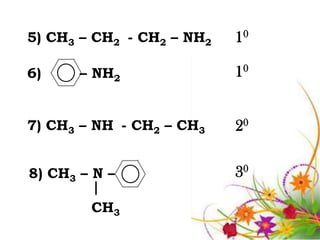
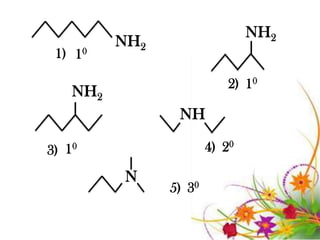
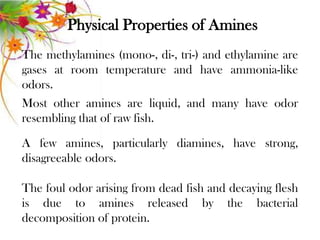
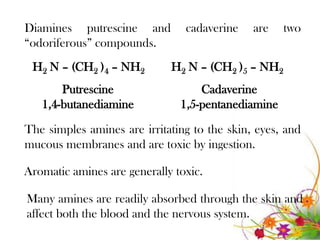
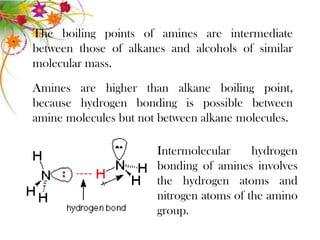
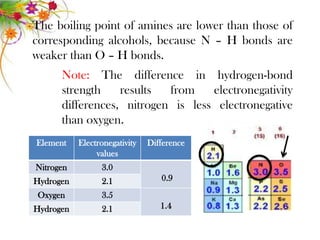
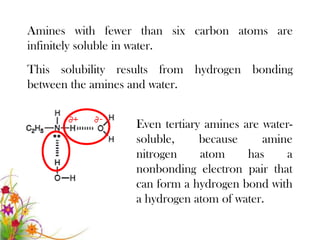
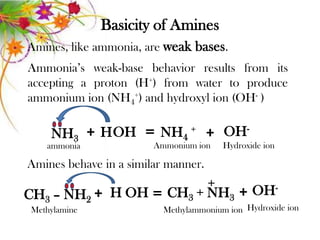
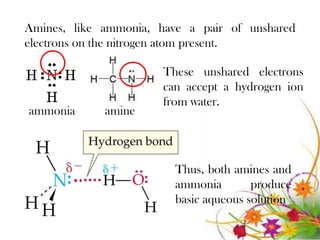
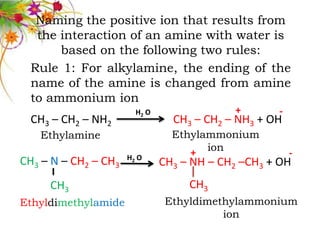
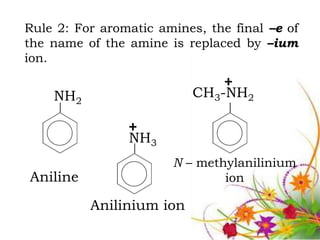
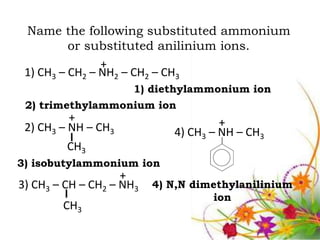
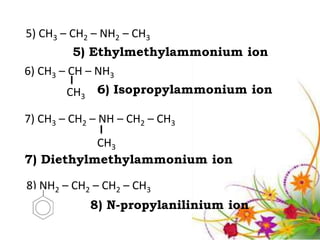
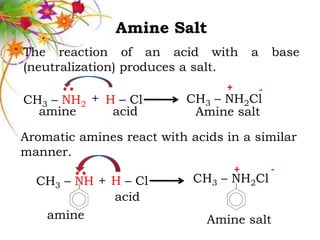
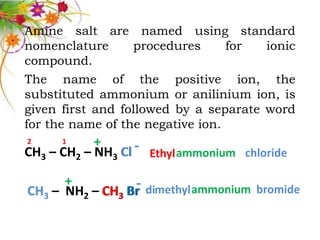
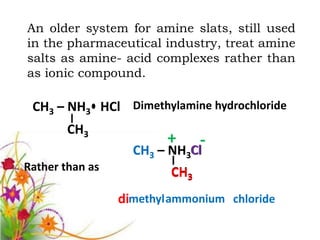
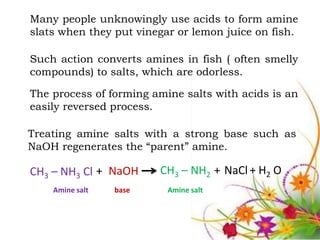
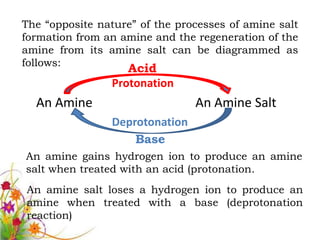
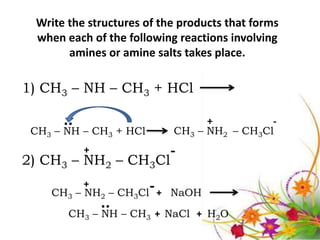
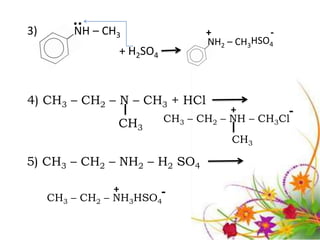
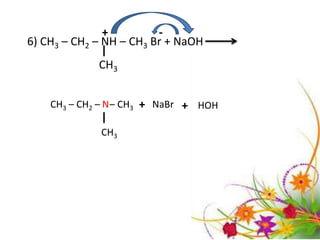
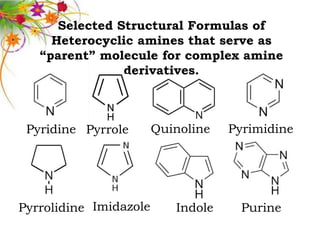
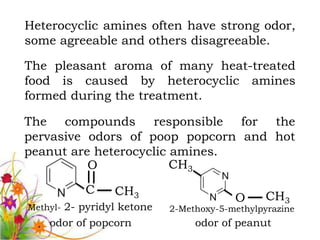
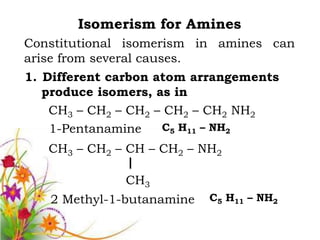
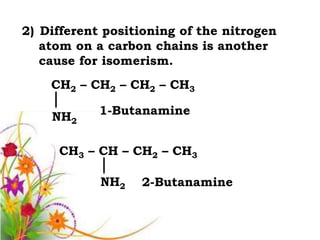
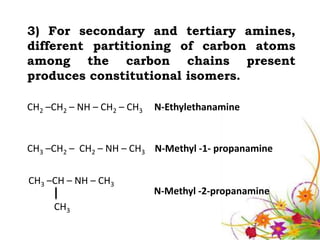
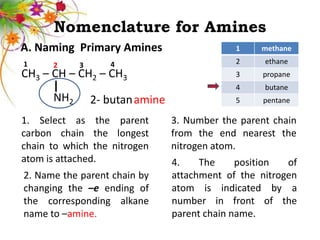
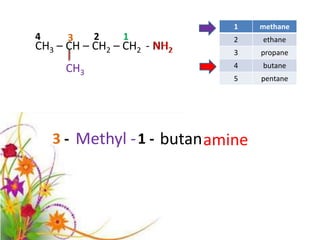
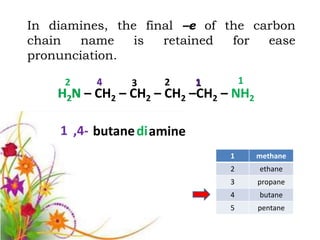
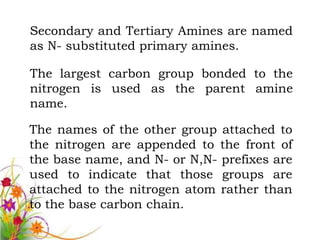
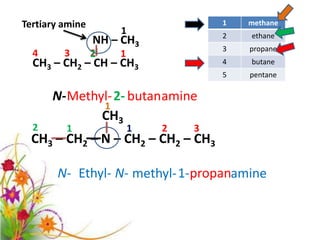
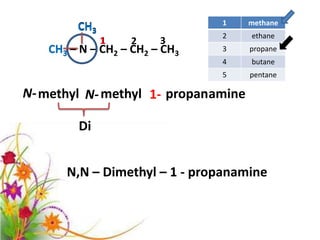
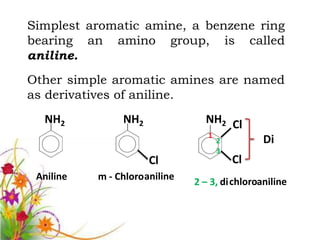
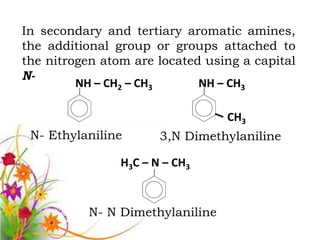
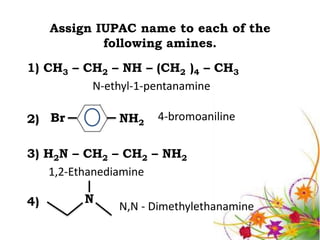
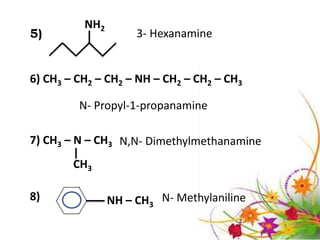
This document discusses the structure and properties of amines. It begins by defining amines as carbon-hydrogen-nitrogen compounds that are commonly found in living organisms. It then discusses the bonding characteristics of nitrogen atoms in organic compounds and how this relates to the structures of primary, secondary, and tertiary amines. The document also covers the physical properties, basicity, and reactions of amines, including the formation of amine salts. It concludes by discussing heterocyclic and isomeric amines.