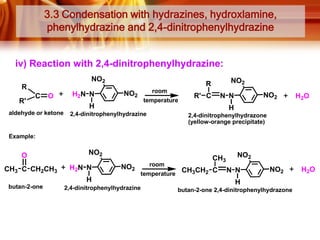
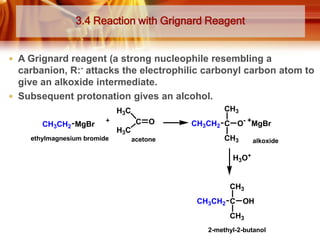
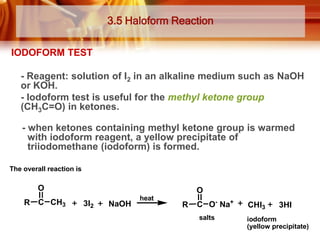
Here are the answers:
a)
i) K (2-methyl-1-propanol):
CH3
|
CH3-C-CH2-CH2-OH
|
CH3
L (2-methyl-2-propanol):
CH3
|
CH3-C-CH(OH)-CH3
ii) K can be prepared by reacting propanone with methylmagnesium bromide, a Grignard reagent:
CH3COCH3 + CH3MgBr → CH3C(OCH3)(CH3) → CH3C(OH)(CH3)CH3 + MgBr
iii) M





![2.1 Oxidation of 2 °Alcohol
• Ketones can be made from 2o alcohols by oxidation
* [O] =
• Examples
14](https://image.slidesharecdn.com/chapter3-ketone-131023012147-phpapp01/85/Chapter-3-ketone-14-320.jpg)


![Tests to Distinguish Aldehydes and Ketones, and Aliphatic Aldehydes and
Aromatic Aldehydes
TESTS
ALDEHYDES
KETONES
Tollens’ Test / silver mirror test
Reagent and condition:
- ammoniacal silver nitrate
solution ([Ag(NH3)2]+)
Observation:
Formation of silver mirror
Observation:
Silver mirror did not formed
* Ketones do not react with
Tollens’ reagent
Fehling’s test / Benedict’s test
Reagent and condition:
-Solution of Cu2+ (aq) ions in an
alkaline solution of sodium
potassium tartate.
Observation;
Blue colour of the Fehling’s
solution dissappears and
brick-red precipitate is
obtained
* Except benzaldehyde
Observation:
Blue colour remains.
* Ketones do not react with
Fehling’s/Benedict’s reagent
Observation:
Formation of magenta-pink
colour (simple aldehydes)
* Except benzaldehyde and
a few aromatic aldehydes)
Observation:
Ketones (except propanone)
do not react with Schiff’s
reagent.
*Can be used to distinguish
between:
i) Aldehydes and ketones
ii) Aliphatic aldehydes and
benzaldehyde
Schiff’s test
Reagent and condition:
- Schiff’s reagent](https://image.slidesharecdn.com/chapter3-ketone-131023012147-phpapp01/85/Chapter-3-ketone-28-320.jpg)



