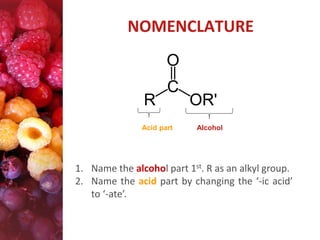
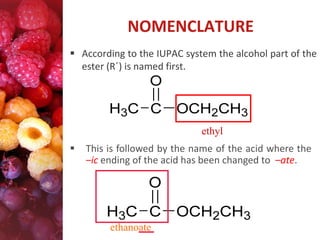
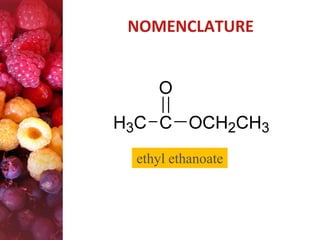
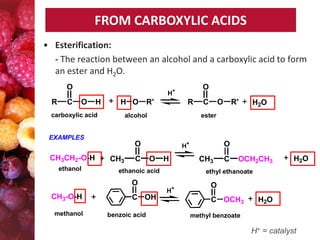
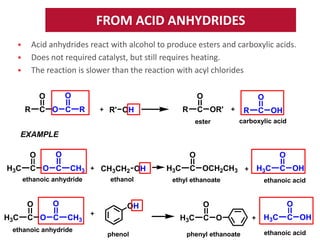
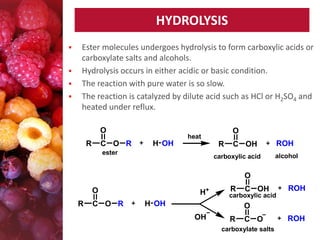
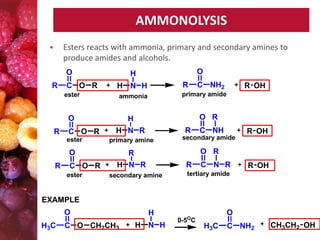
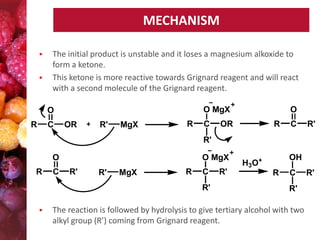
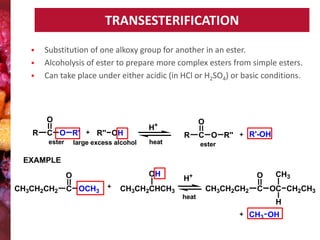
Esters have the general formula RCOOR'. They are formed by the reaction of carboxylic acids with alcohols, which is called esterification. Esters can also be prepared from acyl chlorides or acid anhydrides. Esters undergo various reactions including hydrolysis, aminolysis, reactions with Grignard reagents, and transesterification. Hydrolysis converts esters back into carboxylic acids and alcohols. Transesterification involves exchanging one alkoxy group in an ester for another.