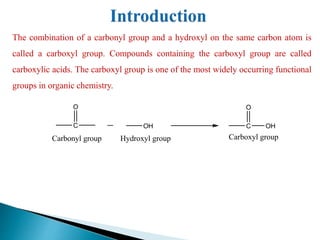
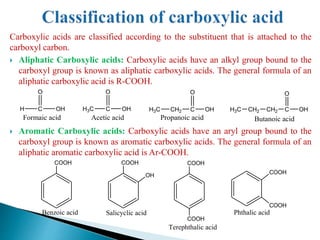
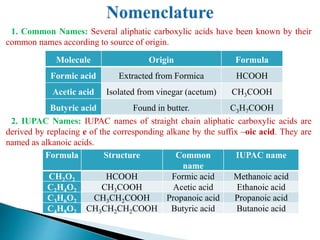
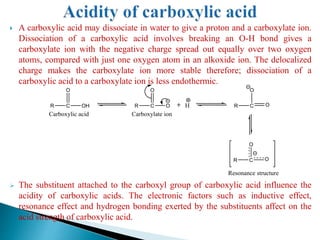
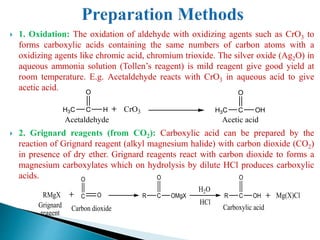
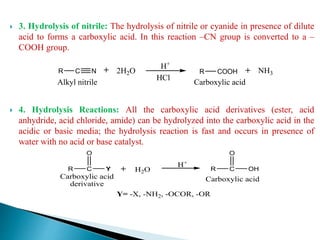
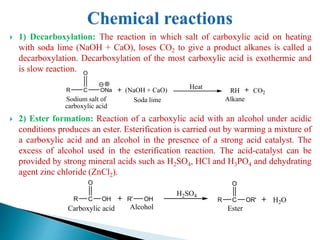
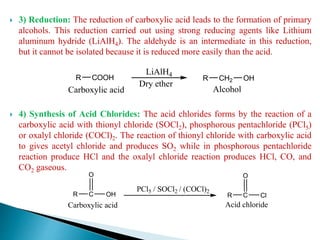
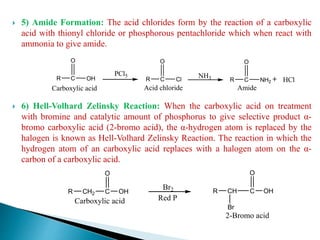
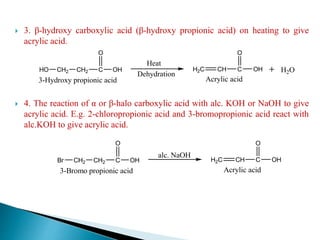
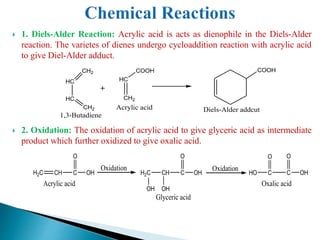
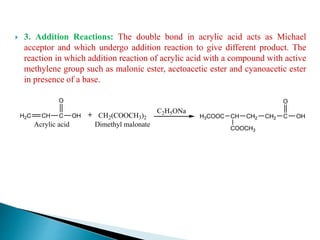
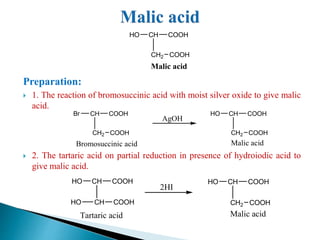
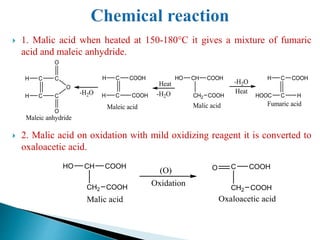
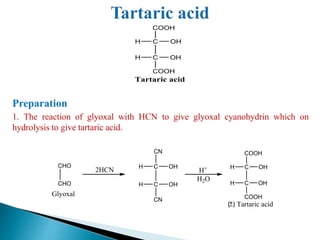
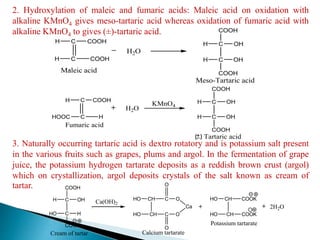
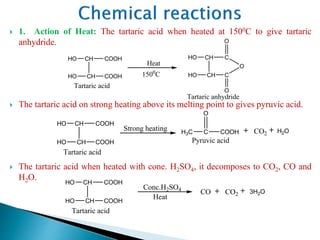
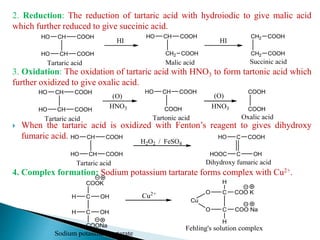
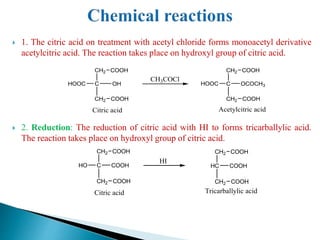
The document provides an overview of carboxylic acids, detailing their structure, classification as either aliphatic or aromatic, and examples of common and IUPAC names. It discusses their properties, synthesis methods, such as oxidation and Grignard reagent reactions, and various chemical reactions they undergo, including ester formation and decarboxylation. Additionally, it covers dicarboxylic acids, particularly malic and tartaric acids, including their interactions and transformations under different conditions.