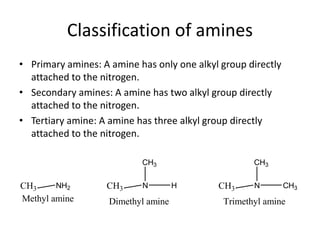
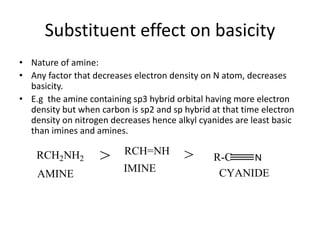
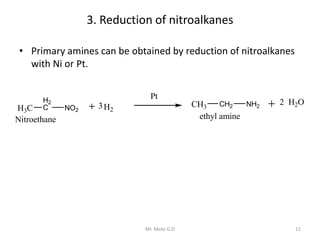
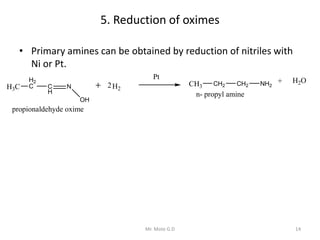
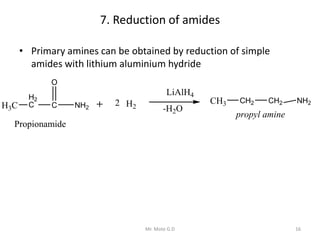
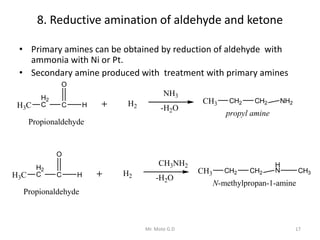
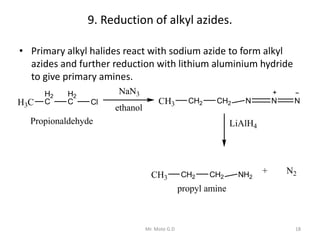
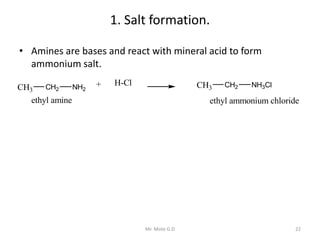
The document provides an extensive overview of amines, including their classification, physical properties, and basicity. It discusses their preparation methods, reactions, and qualitative tests for identification. Additionally, it highlights specific amines and their applications in various fields.