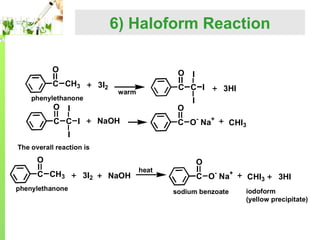
The document provides an overview of aldehydes, including:
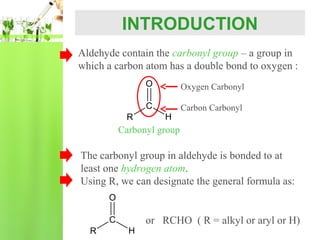
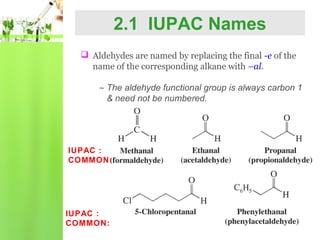
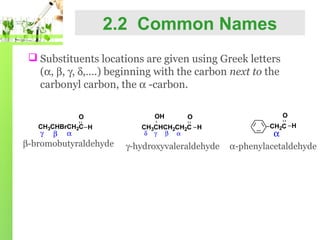
- Nomenclature of aldehydes using IUPAC and common names.
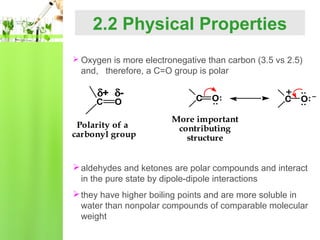
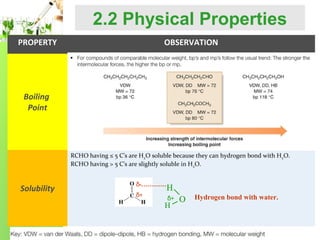
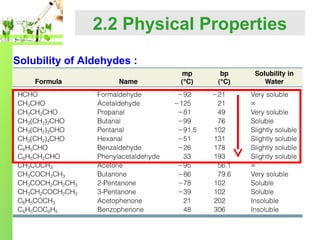
- Physical properties such as boiling points and solubility due to the polar carbonyl group.
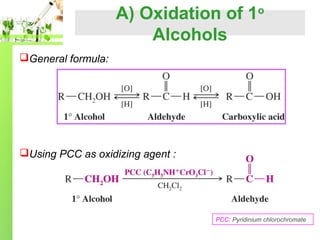
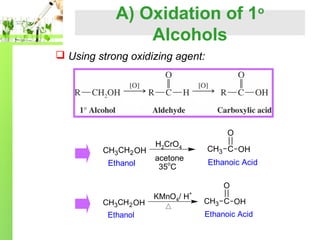
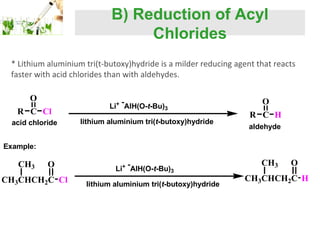
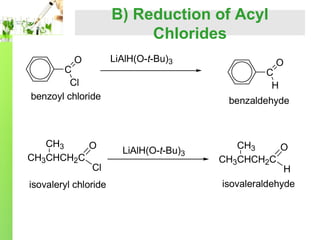
- Preparation methods including oxidation of primary alcohols and reduction of acid chlorides.
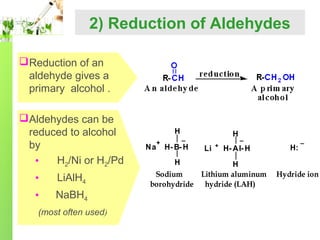
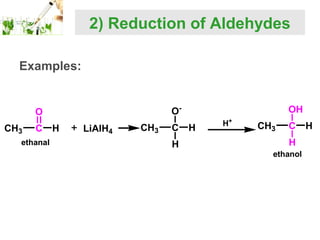
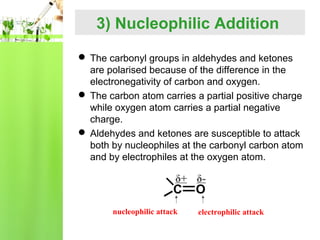
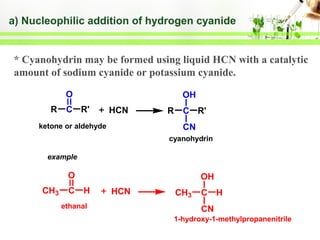
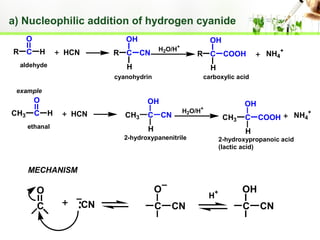
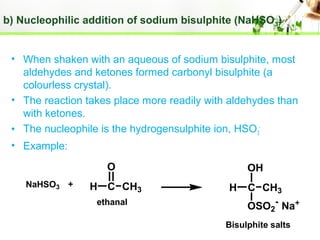
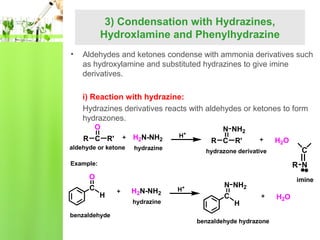
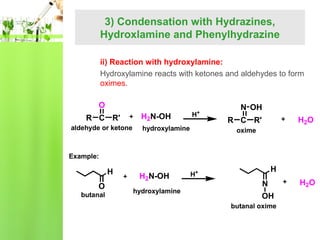
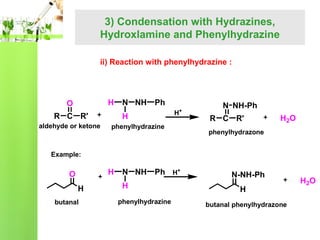
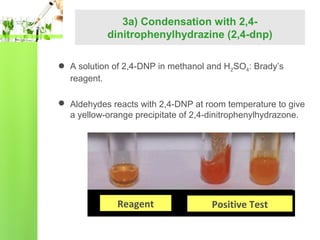
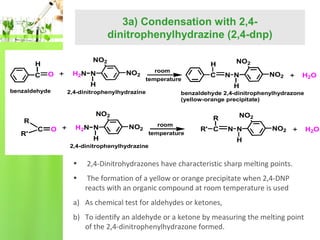
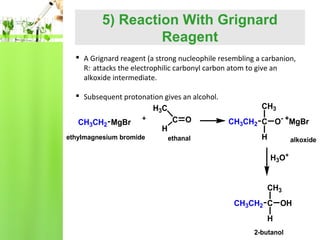
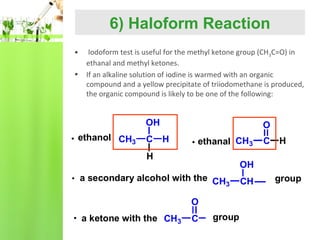
- Reactions including oxidation to carboxylic acids, reduction to alcohols, nucleophilic addition with HCN or Grignard reagents, and condensation reactions with ammonia derivatives.
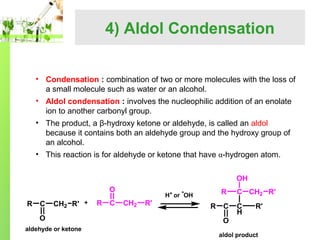
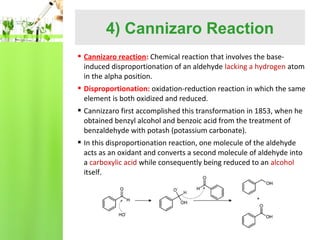
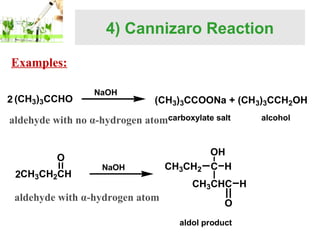
- Specific reactions are discussed in more detail such as cyanohydrin formation, aldol condensation, and Cannizzaro disproportionation.








![1) Oxidation of Aldehydes
Aldehydes are easily oxidized to carboxylic acid by:
strong oxidizing agent such as potassium permanganate,KMnO4
mild oxidizing agent such as silver oxide, Ag2O in aqueous ammonia
(Tollen’s Test : differentiate between aldehyde & ketone)
General Reaction
O
O
[o]
C
R
R
H
OH
[O] :
KMnO4, OHK2Cr2O7/H2SO4
Ag(NH3)2+OH- (Tollen’s reagent)
Carboxylic Acid
Aldehyde
Examples
CH3─ CH2─ CH2─ CH2─ C─ H
O
K2Cr2O7
H2SO4
Pentanal
=
=
O
CH3─ CH2─ CH2─ CH2─ C─ OH
Pentanoic acid](https://image.slidesharecdn.com/chapter2-aldehyde-131023012133-phpapp02/85/Chapter-2-aldehyde-20-320.jpg)