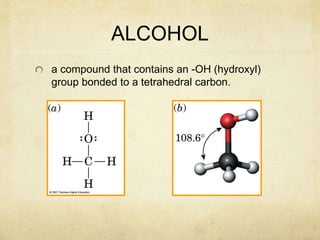
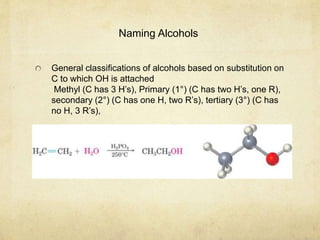
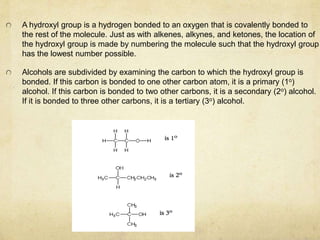
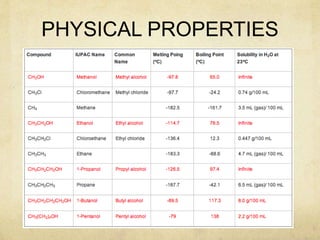
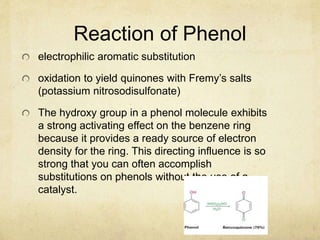
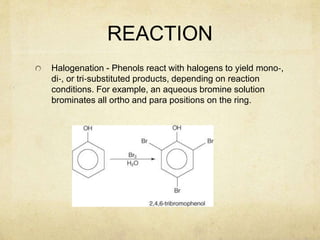
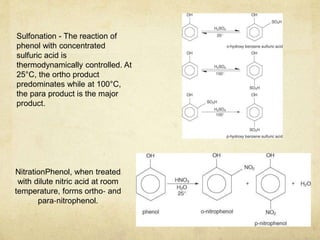
Alcohols contain an -OH group bonded to a carbon atom. They are classified based on the carbon the OH group is attached to as primary, secondary, or tertiary alcohols. Alcohols have higher boiling points than similar hydrocarbons due to hydrogen bonding. Common alcohols include methanol, ethanol, and isopropyl alcohol. Alcohols are used in drinks, fuels, solvents, and to synthesize other organic compounds. Phenol contains an OH group bonded directly to a benzene ring. It is used as an antiseptic and in making resins, plastics, and pharmaceuticals. Phenol undergoes electrophilic aromatic substitution reactions more readily than benz