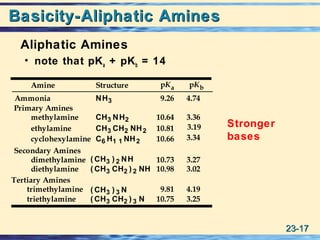
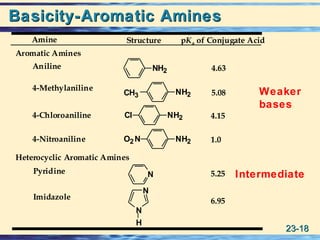
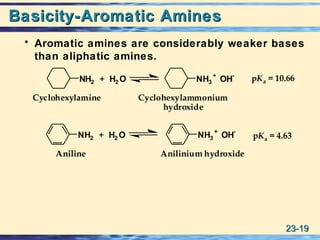
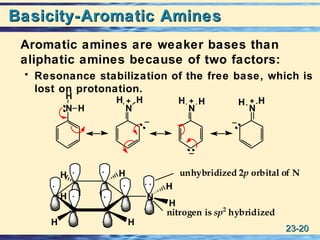
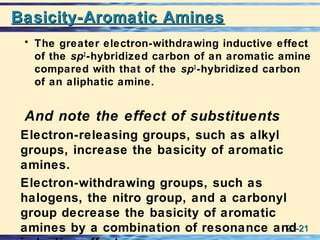
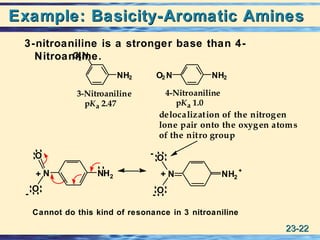
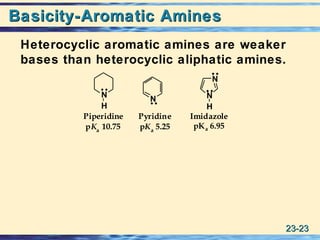
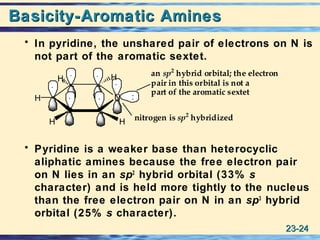
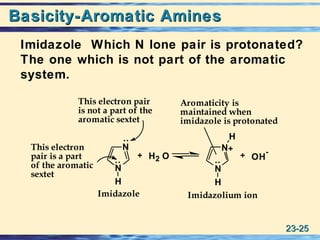
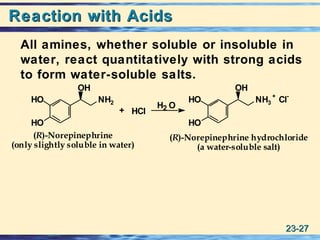
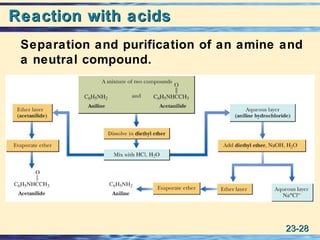
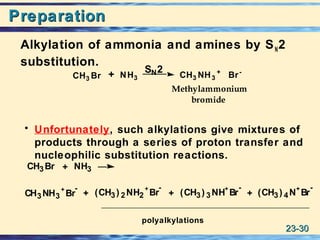
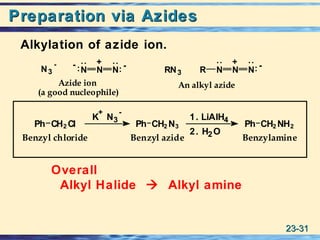
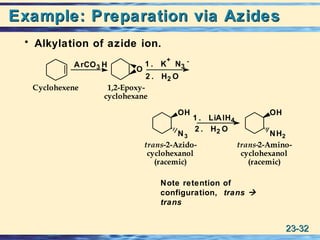
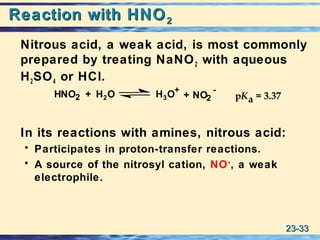
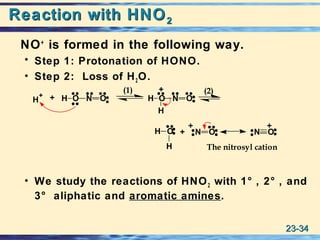
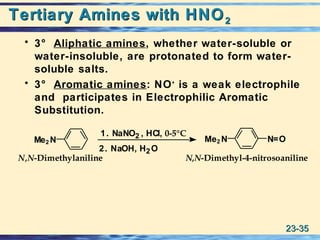
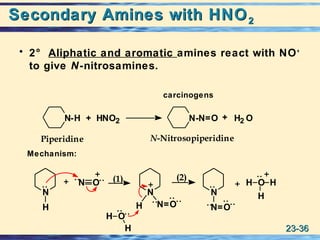
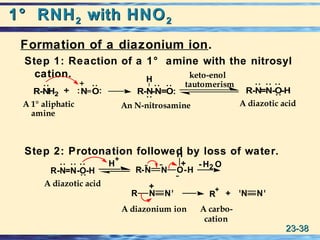
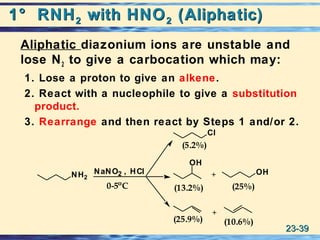
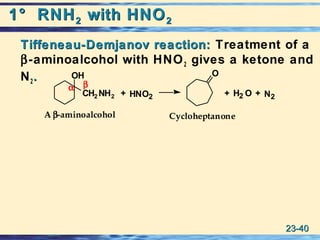
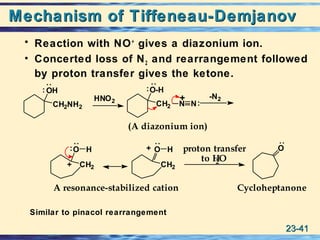
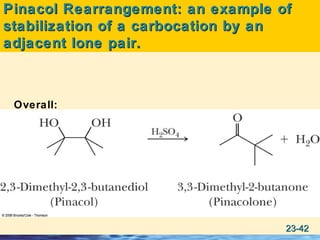
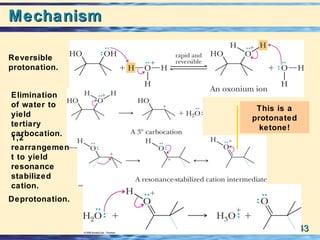
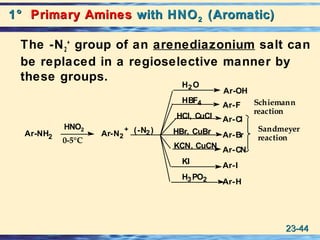
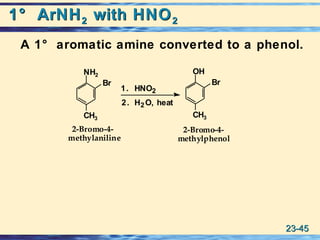
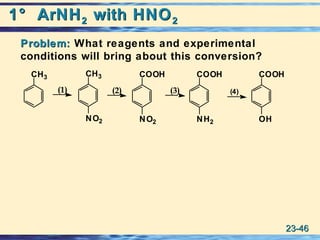
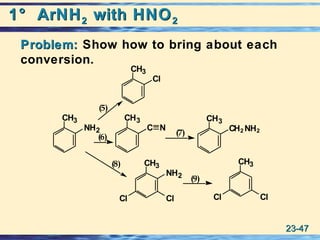
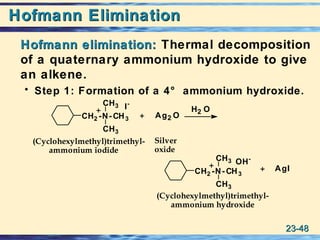
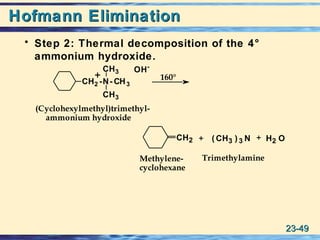
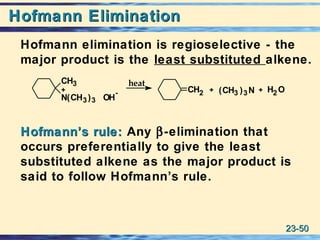
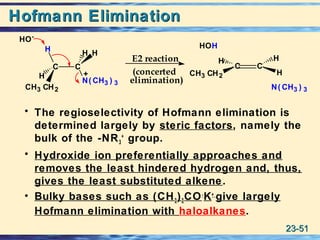
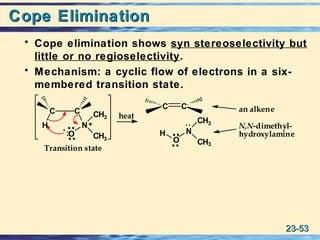
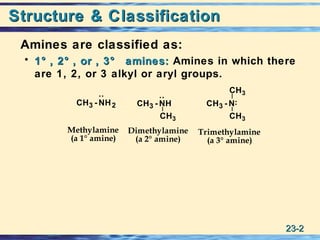
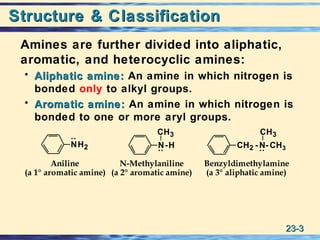
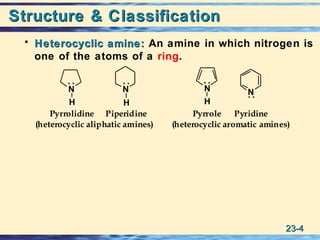
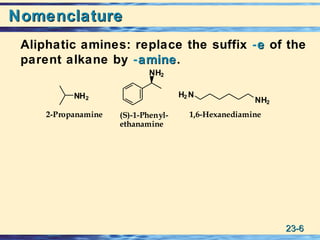
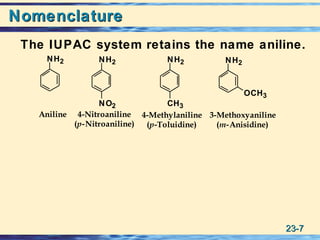
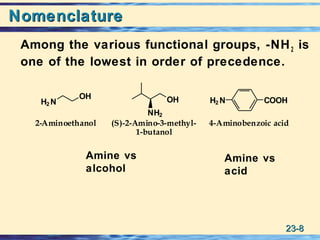
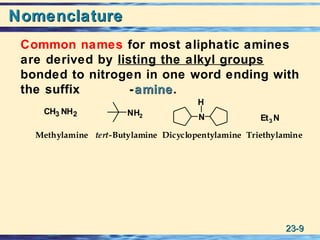
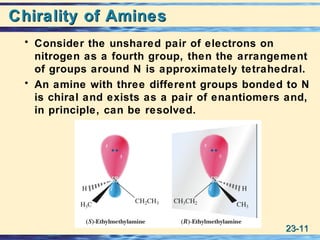
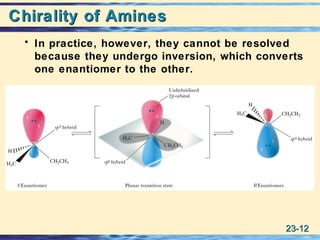
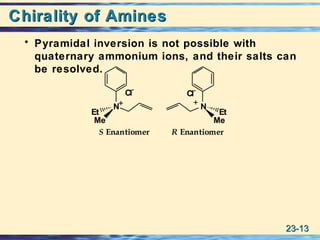
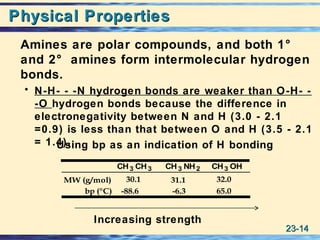
The document discusses the classification, nomenclature, properties, preparation, and reactions of amines. Amines are classified as primary, secondary, or tertiary depending on the number of alkyl or aryl groups bonded to the nitrogen atom. They are further divided into aliphatic, aromatic, and heterocyclic amines. Amines are named according to IUPAC nomenclature rules. They are weak bases due to resonance stabilization of the conjugate acid. Common methods for preparing amines include reduction of nitriles, amides, imines, and nitro compounds. Amines react with acids to form water-soluble salts and with nitrous acid to undergo proton-transfer and electrophilic aromatic substitution reactions.

![23-23-1515
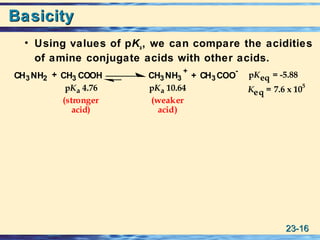
BasicityBasicity
All amines are weak bases, and aqueous
solutions of amines are basic.
• It is common to discuss their basicity by
reference to the acid ionization constant of the
conjugate acid.
CH3NH3
+
H2 O CH3 NH2 H3 O+++
[ CH3NH2] [H3 O
+
]
[CH3 NH3
+
]
2.29 x 10
-11
==Ka pKa = 10.64
H
H
CH3 - N H- O-H
H
H
CH3 - N- H O-H
Methylammonium hydroxideMethylamine
+
+
-](https://image.slidesharecdn.com/23amines-160131014750/85/amines-14-320.jpg)