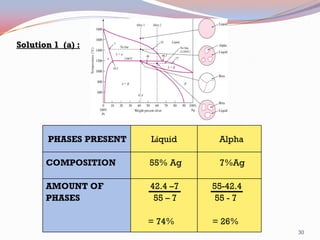
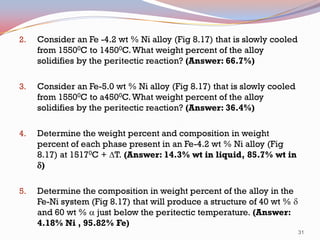
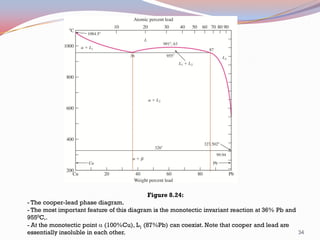
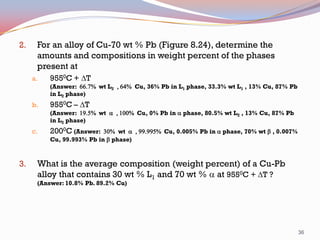
The document provides information on phase diagrams including:
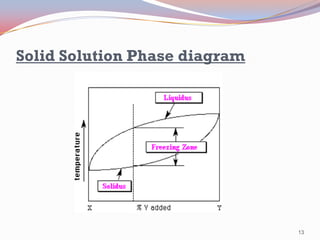
- Phase diagrams represent the phases present in materials at different conditions of temperature, pressure, and composition. They indicate solubility, solidification ranges, and melting points.
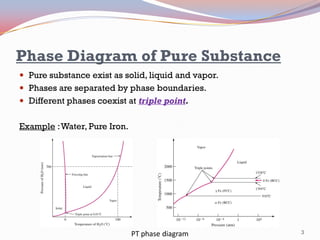
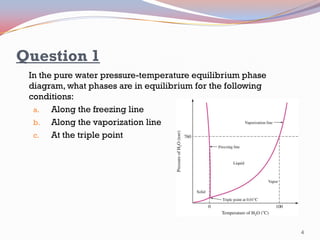
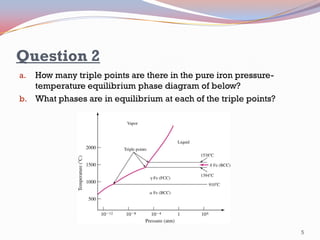
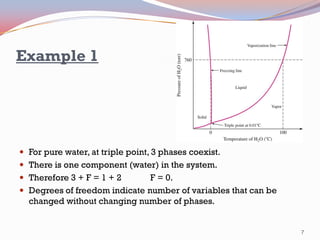
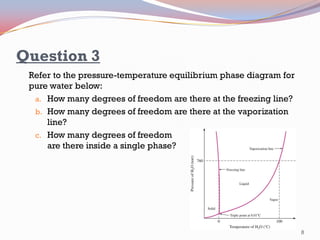
- Pure substances have solid, liquid, and vapor phases separated by phase boundaries and coexisting at triple points, as shown in pressure-temperature diagrams.
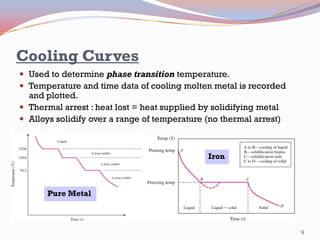
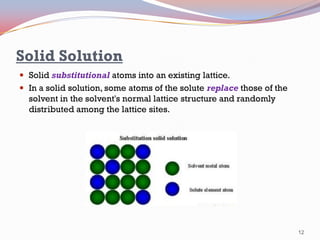
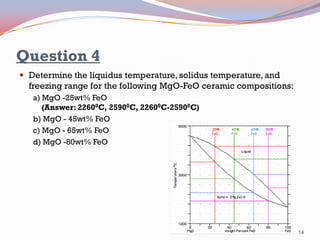
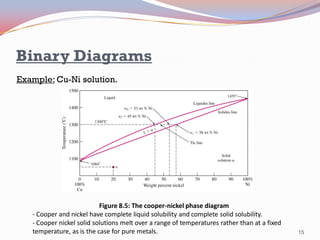
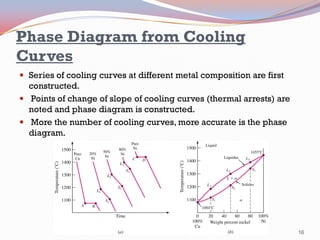
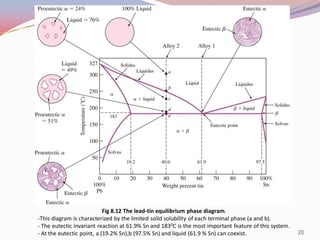
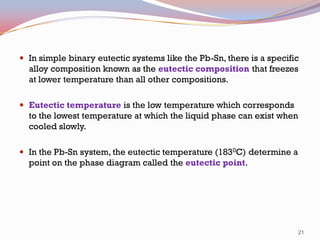
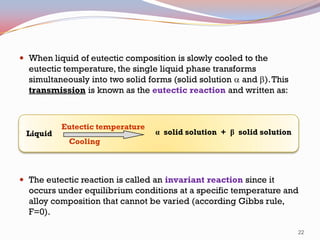
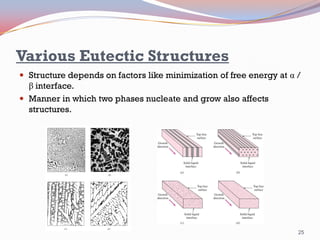
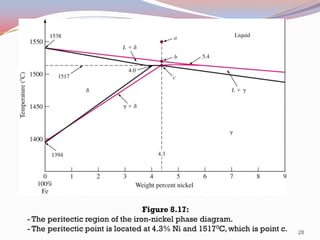
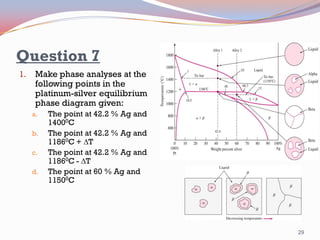
- Binary alloy phase diagrams show the phases present at different compositions and temperatures, including solid solutions, eutectic points where two solids form from liquid, and peritectic reactions where a solid and liquid form a new solid phase.
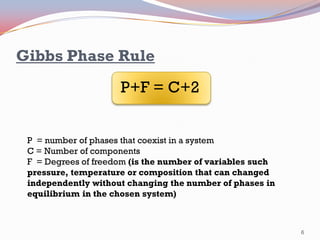
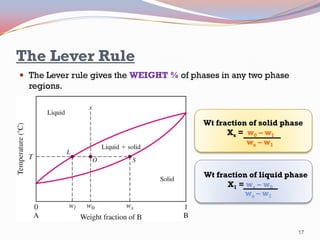
- The Gibbs phase rule and lever rule are used to analyze multi-phase regions. Cool