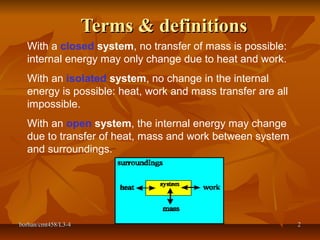
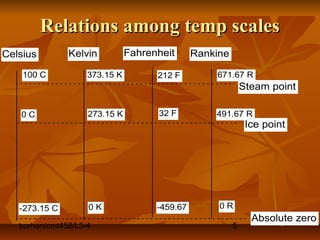
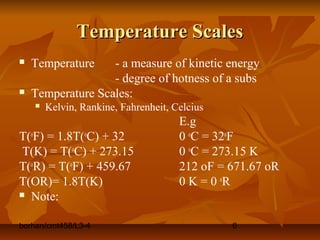
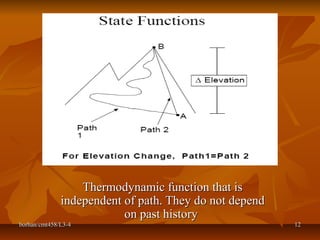
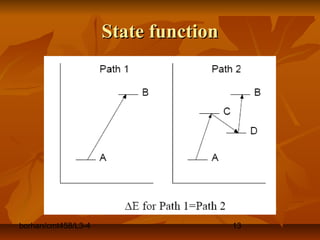
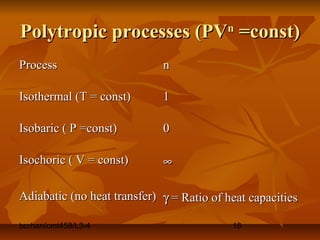
This document defines key terms and concepts in thermodynamics. It discusses systems, surroundings, and the universe. It describes closed, isolated, and open systems. It also defines intensive and extensive properties, homogeneous and heterogeneous systems, temperature scales, states of matter, state and path functions, processes like isothermal and adiabatic, and equilibrium.