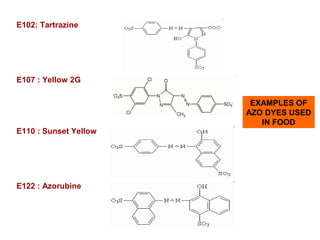
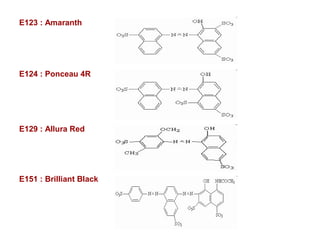
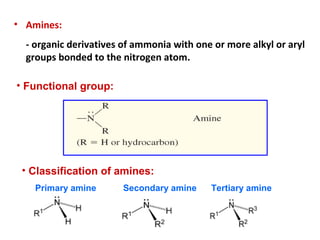
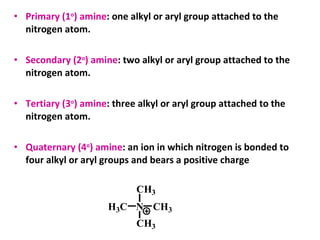
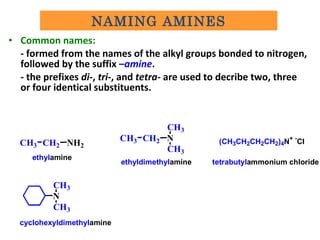
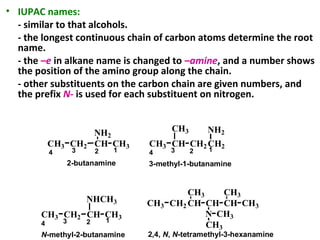
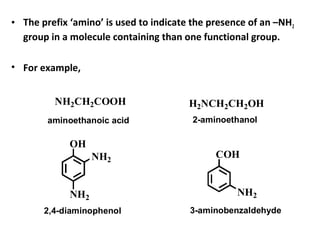
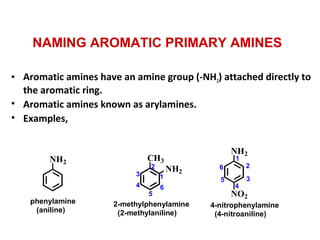
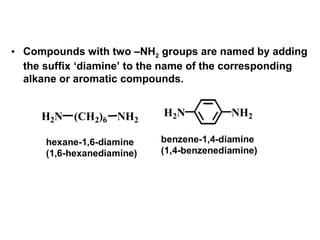
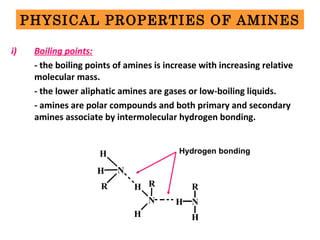
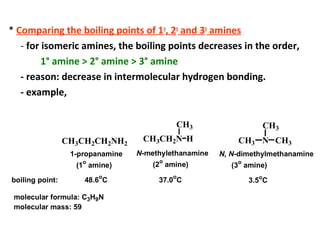
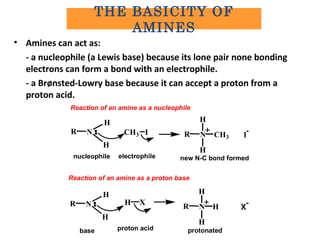
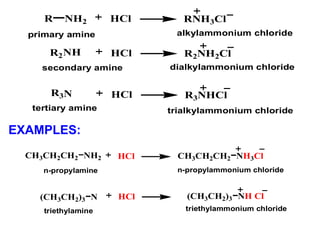
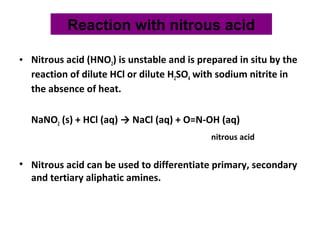
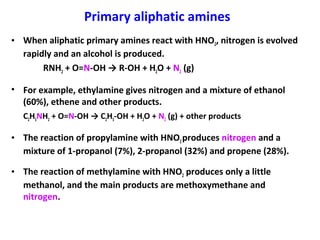
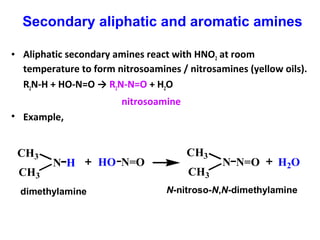
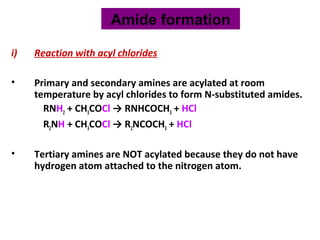
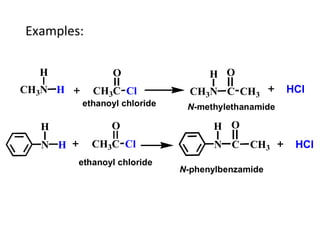
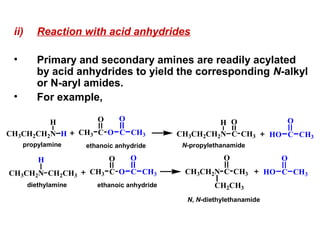
Amines are organic compounds derived from ammonia by replacing one or more hydrogen atoms with alkyl or aryl groups. They can be classified as primary, secondary, or tertiary depending on the number of alkyl/aryl groups attached to the nitrogen atom. Amines exhibit hydrogen bonding and have higher boiling points than comparable alkanes. They act as bases and react with acids to form salts. Primary amines react with nitrous acid to form nitrogen gas and alcohols/alkenes, while secondary amines form nitrosamines. Aromatic amines are used to synthesize azo dyes through diazonium salt formation. Nylon is produced by condensation polymerization of diamines and dic



![• Amines are fairly strong base and their aqueous solutions are basic.
• An amine can abstract a proton from water, giving an ammonium
ion and a hydroxide ion.
• The equilibrium constant for this reaction is called base-dissociation
constant, symbolized by Kb.
NR
H
H
OH NR
H
H
H OH
-
H
Kb
Kb = [RNH3
+
] [-
OH]
[RNH2]
pKb = - log 10 Kb
Stronger base have smaller values of pKb](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-14-320.jpg)




![• A tertiary aliphatic amines react with HNO2 will produced
ammonium salts which is dissolve readily in water as a clear
solution.
R3N + HNO2 → [R3NH]+
NO2
-
(aq)
NH2 HNO2 HCl N2
+
Cl
- 2H2O
RT
RT = room temperature
N2
5o
C
benzenediazonium chloride
mixture
products
Tertiary aliphatic amines
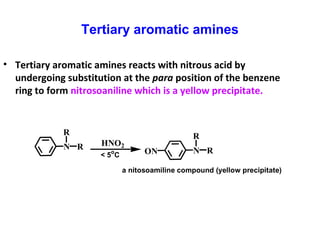
Primary aromatic amines
• A primary aromatic amines react with cold HNO2 and
dissolved in dilute HCl at 0-5o
C will produced diazonium
salt. When this cold salts heated at room temperature,
nitrogen gas will evolved.](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-23-320.jpg)
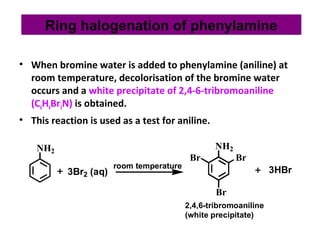
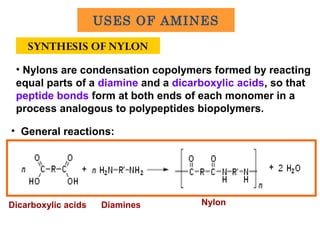
![Basic concepts of nylon production
• The first approach:
- combining molecules with an acid (COOH) group on each
end are reacted with two chemicals that contain amine (NH2)
groups on each end.
- Form nylon 6,6, made of hexamethylene diamine with six
carbon atoms and acidipic acid, as well as six carbon atoms.
• The second approach:
- a compound has an acid at one end and an amine at the
other and is polymerized to form a chain with repeating units
of (-NH-[CH2]n-CO-)x.
- Form nylon 6, made from a single six-carbon substance
called caprolactam.](https://image.slidesharecdn.com/chapter-9-amines-130907082415-/85/Chapter-9-amines-30-320.jpg)