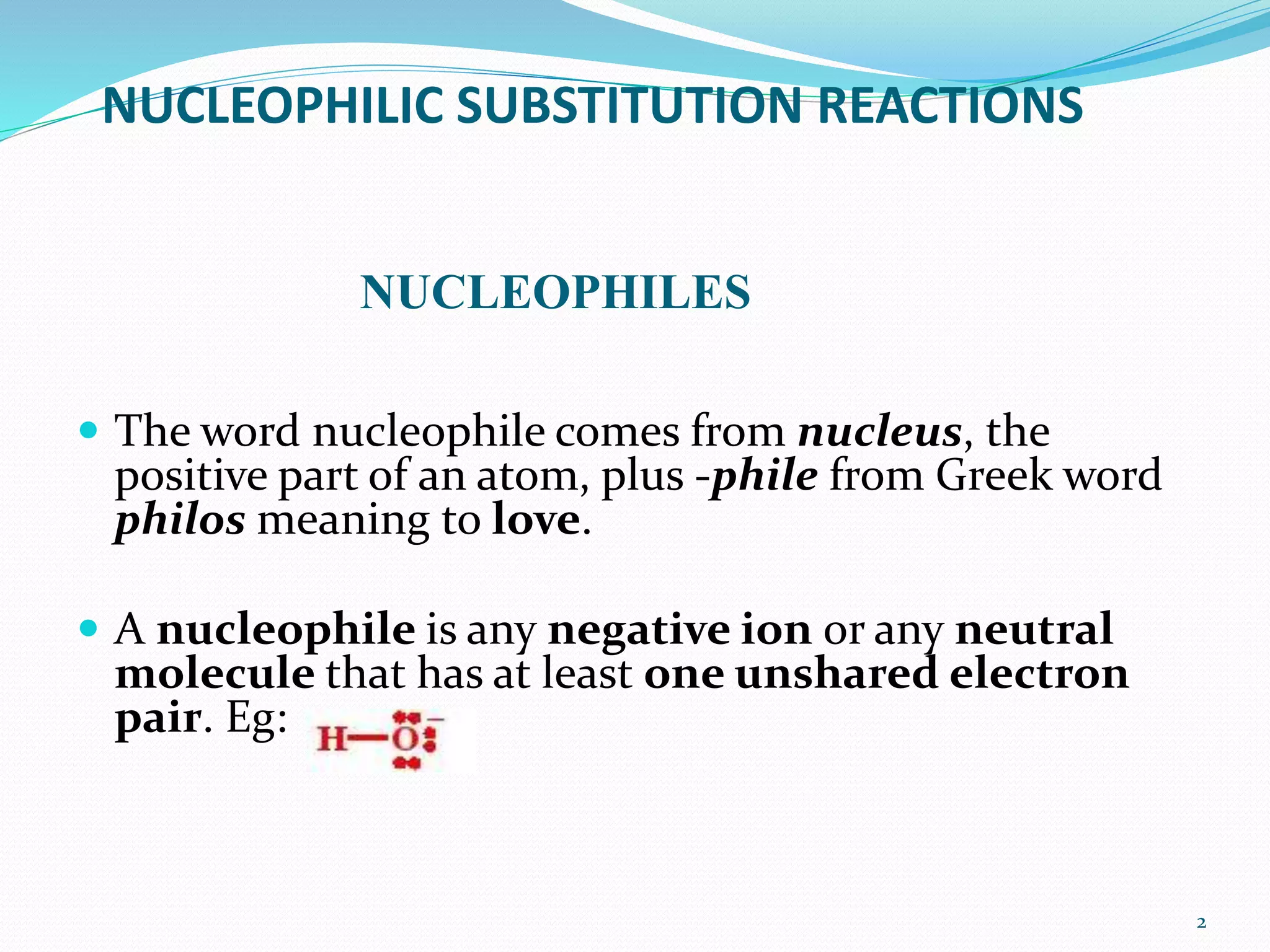
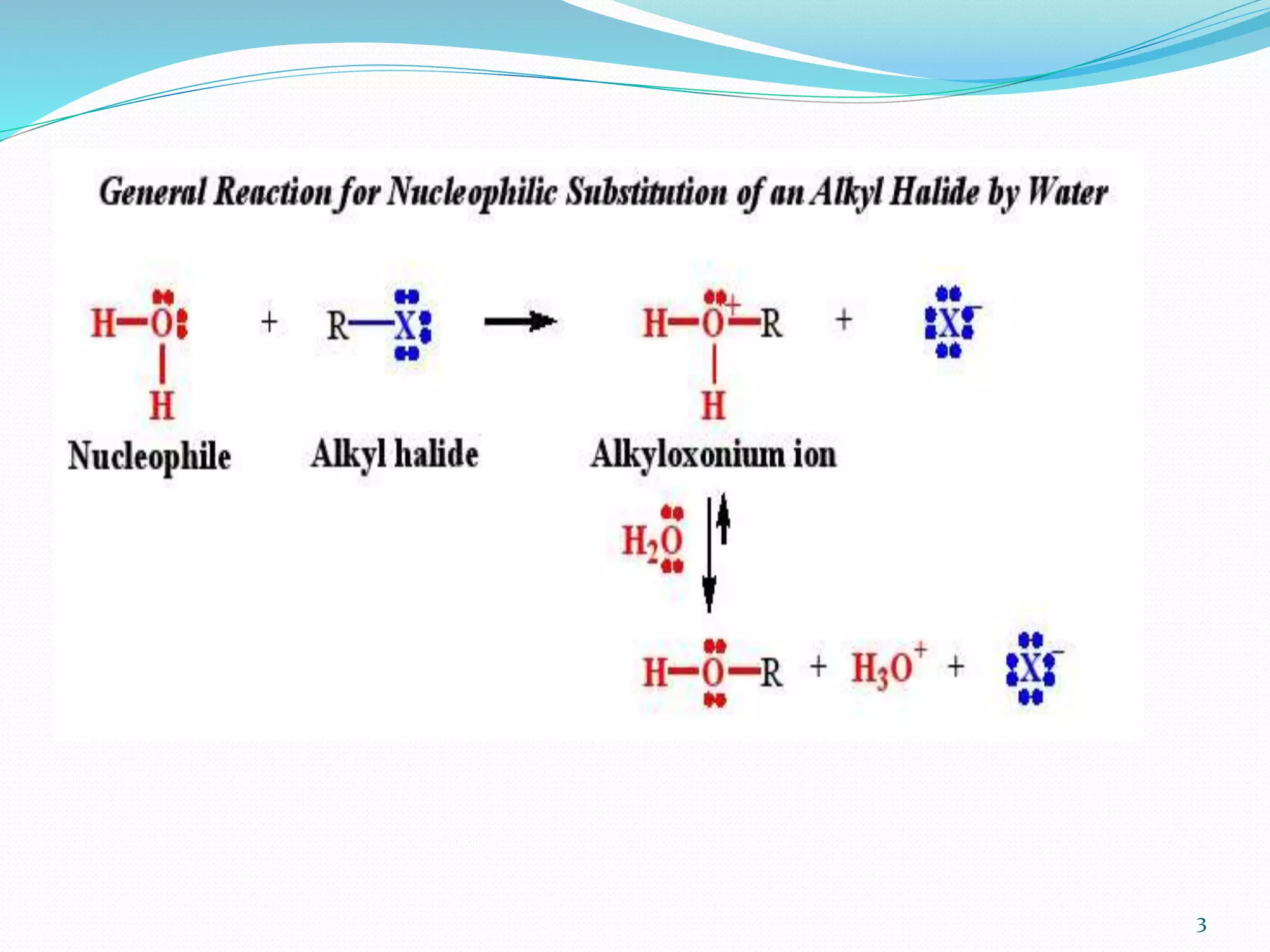
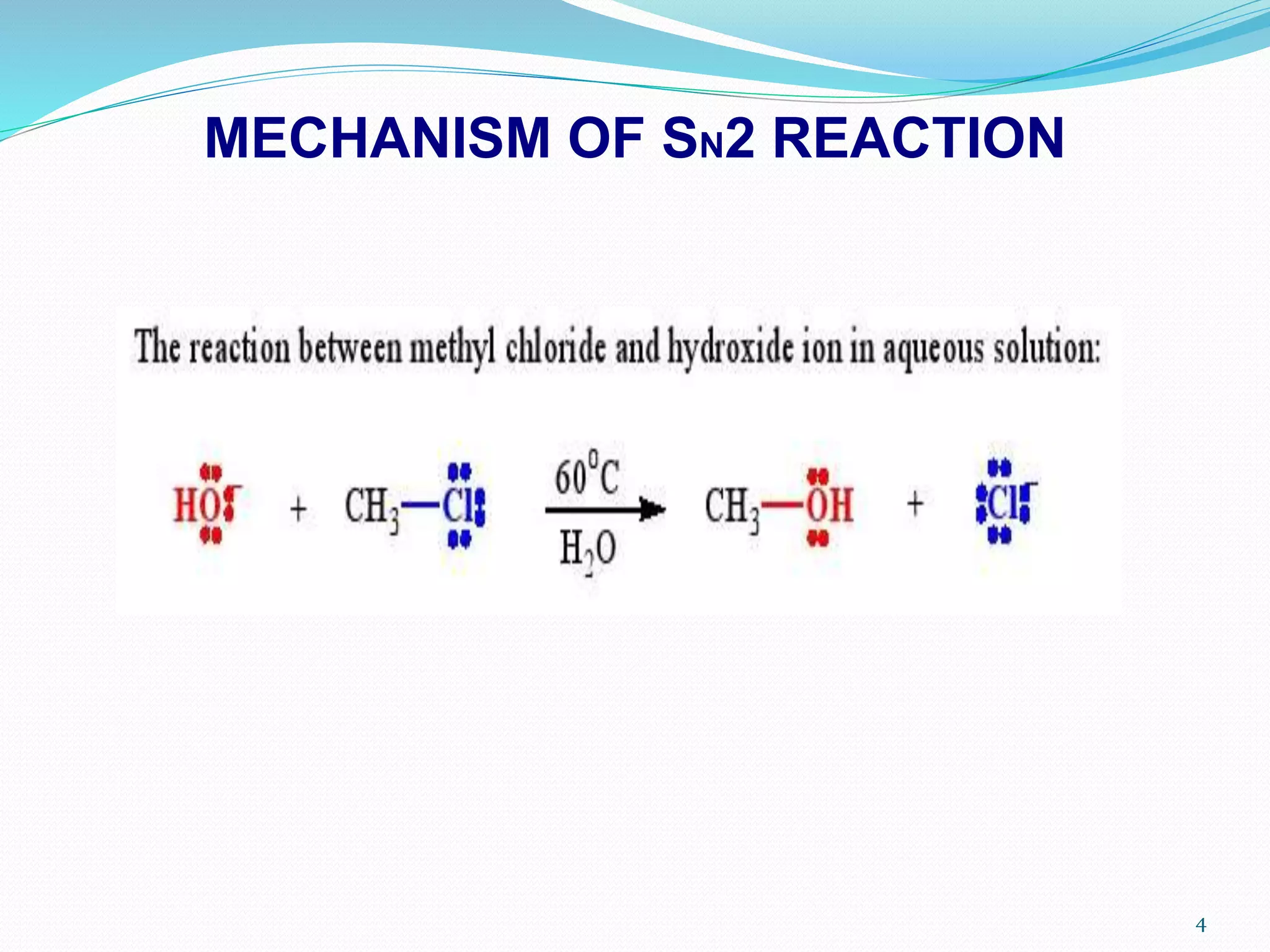
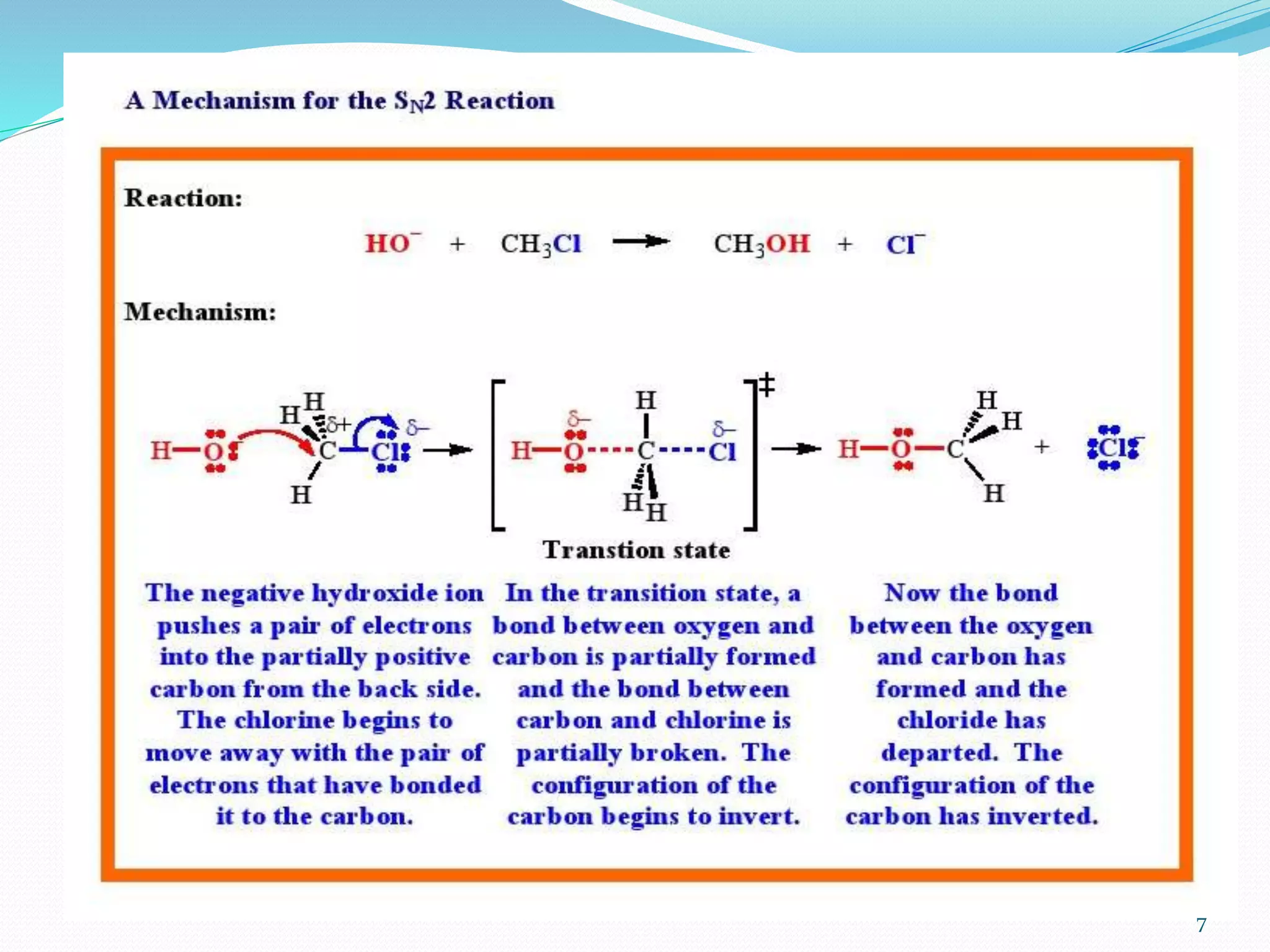
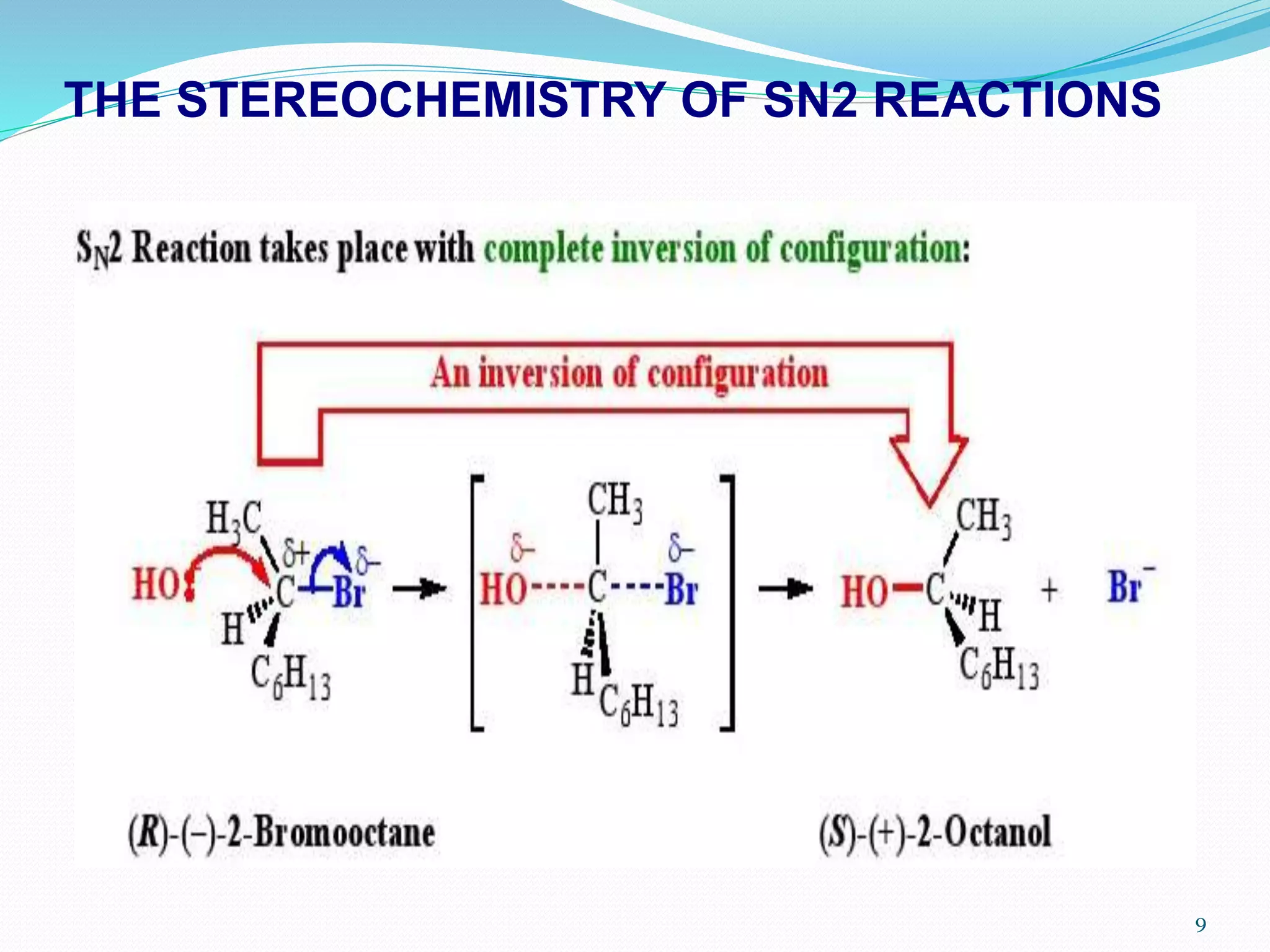
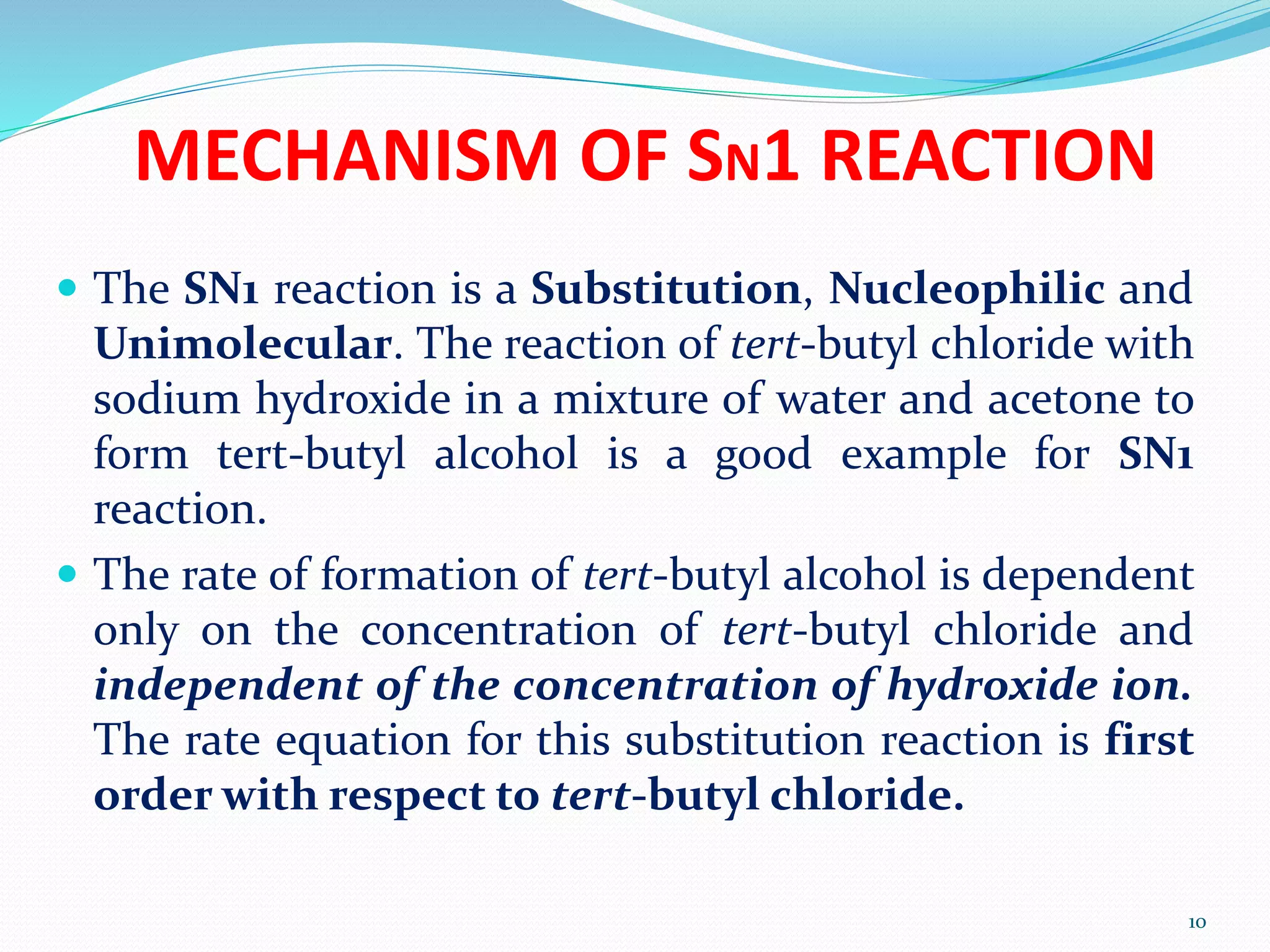
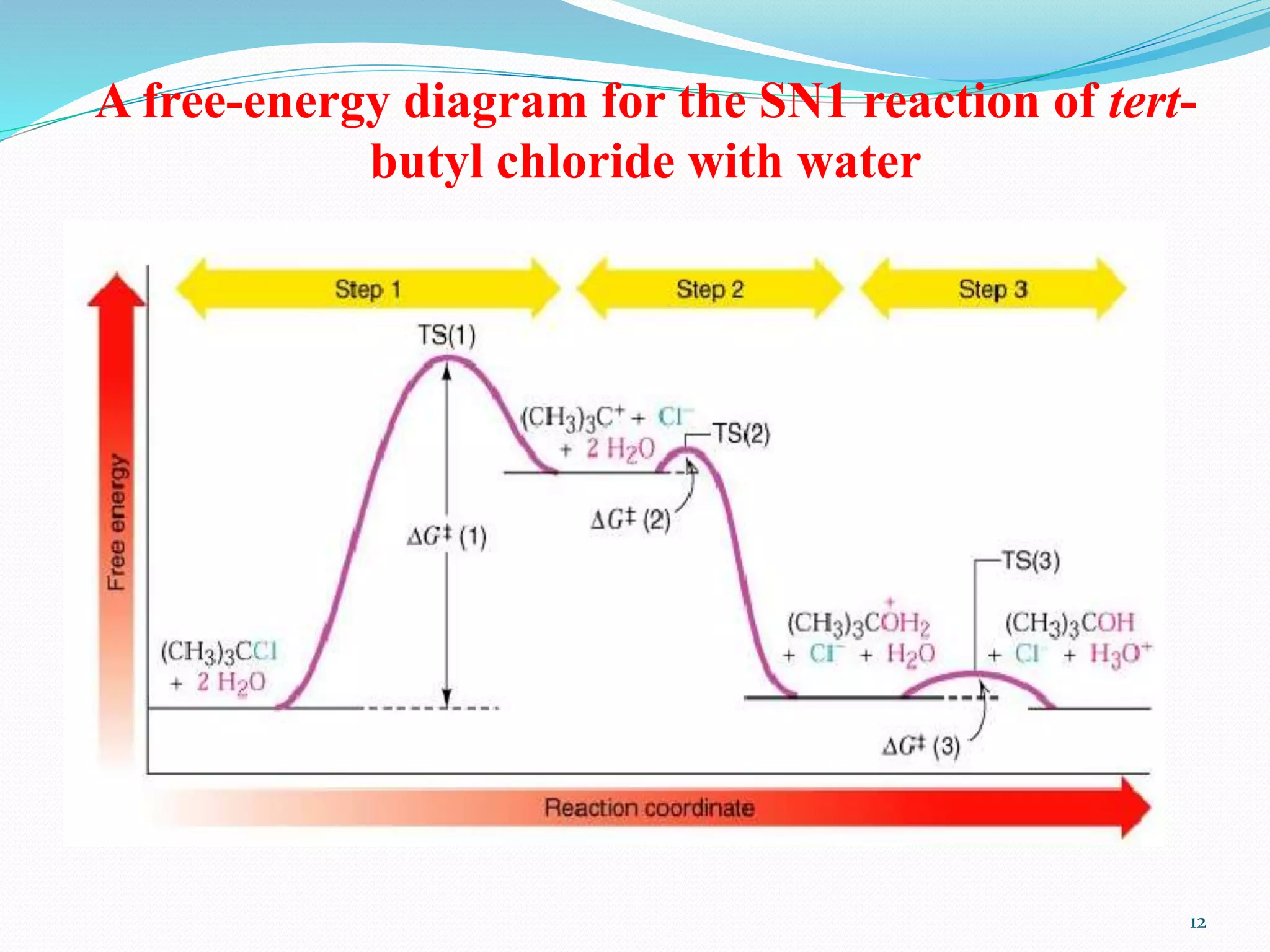
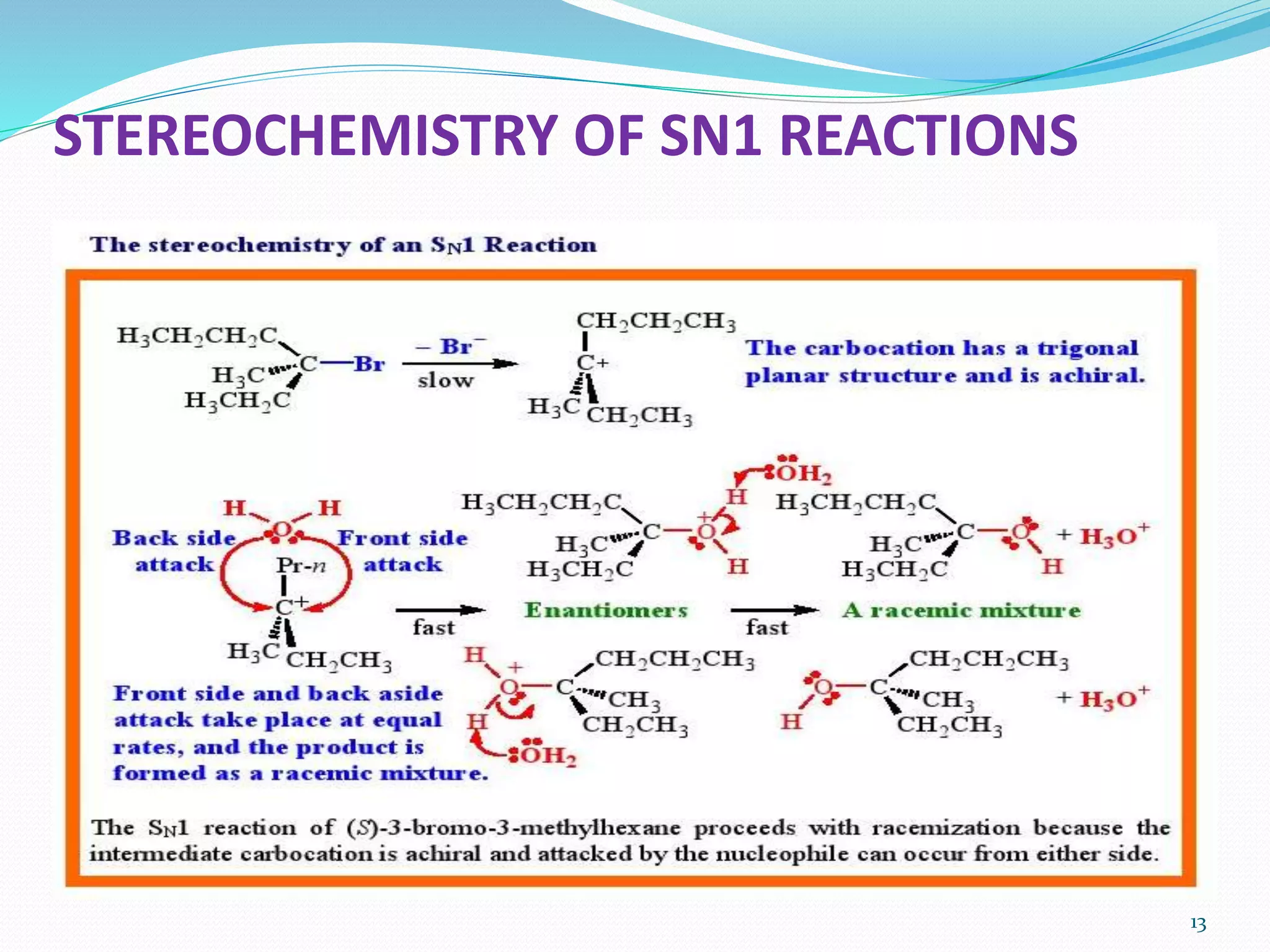
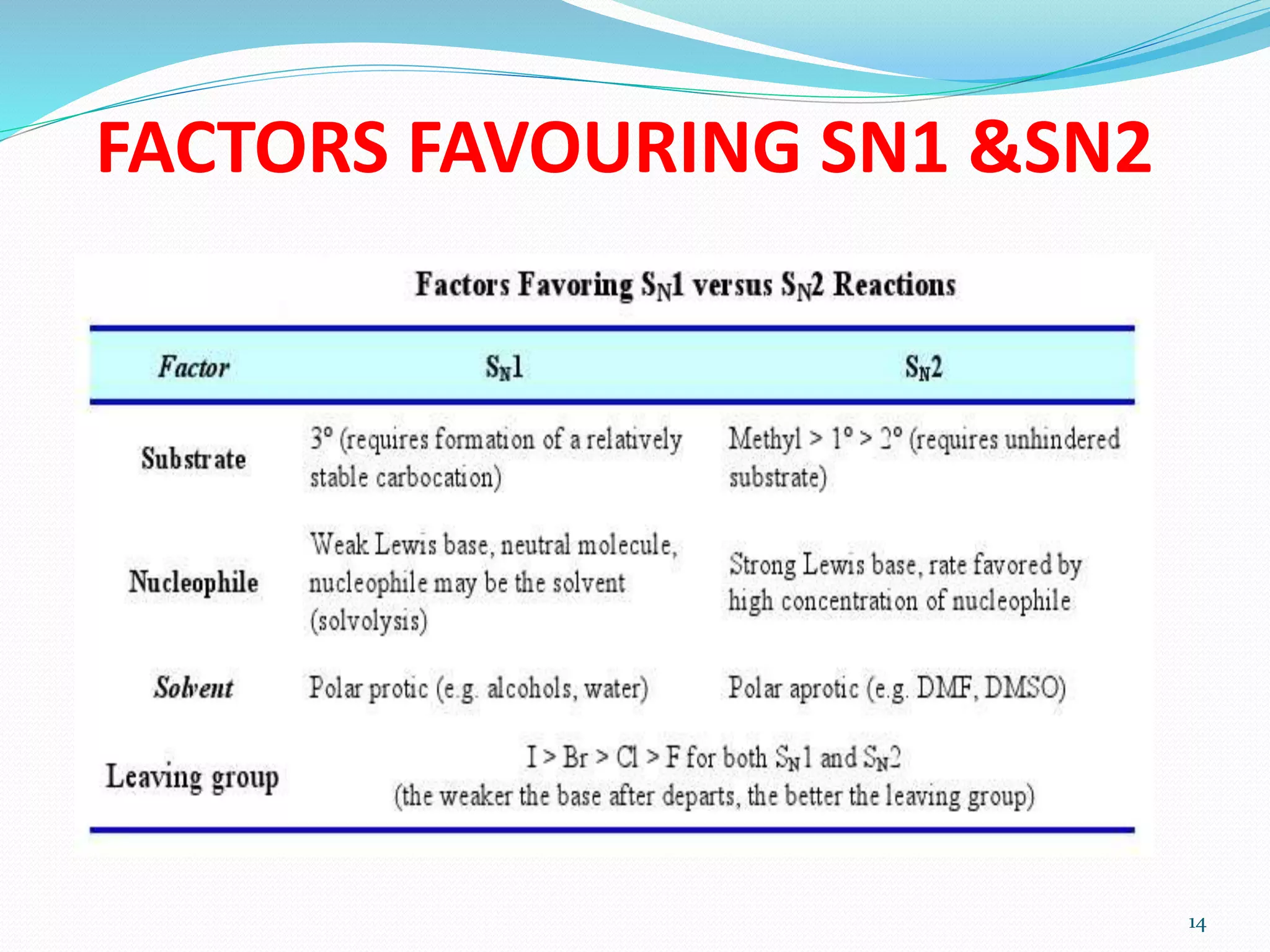
This document discusses nucleophilic substitution reactions. It begins by defining nucleophiles as negatively charged ions or neutral molecules with a lone pair of electrons. It then explains the mechanisms of the SN2 and SN1 reactions. The SN2 is a concerted bimolecular reaction where the nucleophile attacks from the backside of the substrate, inverting the configuration. The SN1 is a unimolecular reaction that proceeds through a carbocation intermediate, allowing for retention or inversion of configuration. Finally, it discusses factors like temperature, nucleophile strength, and substrate structure that determine whether a reaction will proceed by SN1 or SN2.