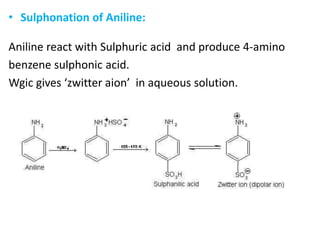
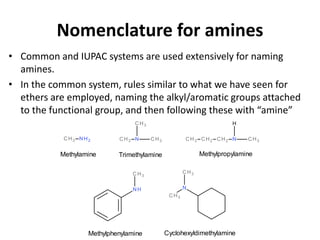
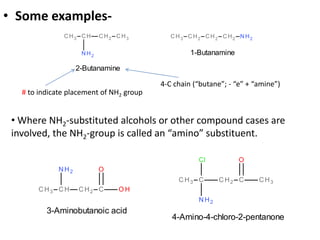
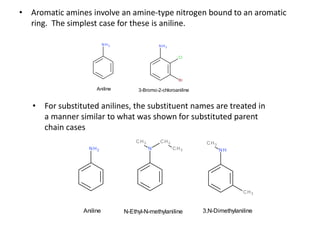
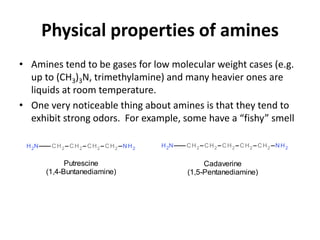
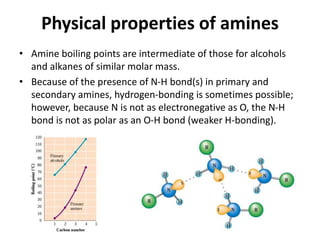
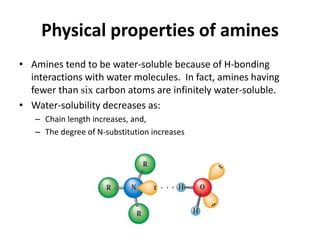
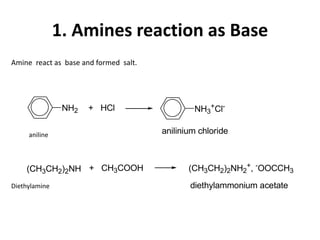
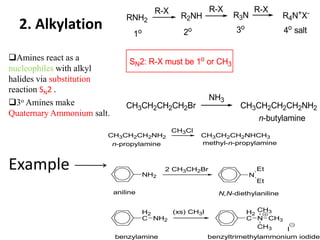
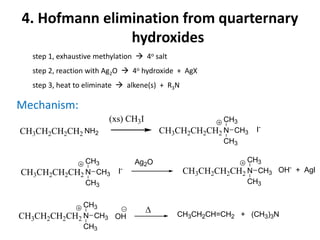
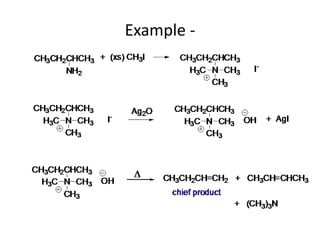
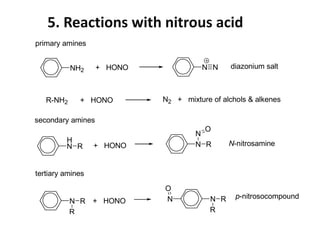
Amines are organic derivatives of ammonia categorized into primary, secondary, and tertiary based on the number of alkyl or aromatic groups attached to the nitrogen. The document discusses their nomenclature, physical properties such as solubility and boiling points, as well as their chemical reactions including basicity, alkylation, and various reactions involving acids and alkyl halides. Key examples and mechanisms of reactions are also provided, highlighting the significant role of amines in organic chemistry.

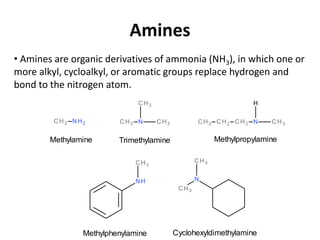
![Classification of Amines
Classified as primary, secondary and tertiary
[according to the number of groups attached to the
nitrogen atom].
1o amine 2o amine 3o amine](https://image.slidesharecdn.com/aminesintroclassficationnomenclaturephysicalpropertiesreactions-170722060235/85/Amines-3-320.jpg)



![R-NH2 + RCOCl RCONHR + HCl
1o N-substituted. amide
R2NH + RCOCl RCONR2 + HCl
2o N,N-disubstituted. amide
R3N + RCOCl No reaction
[ cause No Substitution Hydrogen]
3o
3. Conversion into Amide
NH2 + (CH3CO)2O
H
N C CH3
O
N-phenylacetamide
C
O
Cl
(CH3CH2)2NH + C
O
N CH2CH3
CH2CH3
N.N-diethyl-m-toluamide
N CH3
CH3
+ CH3C
O
Cl
NR
H3C H3C
DEET
Example](https://image.slidesharecdn.com/aminesintroclassficationnomenclaturephysicalpropertiesreactions-170722060235/85/Amines-16-320.jpg)
![6. Carbylamine reaction
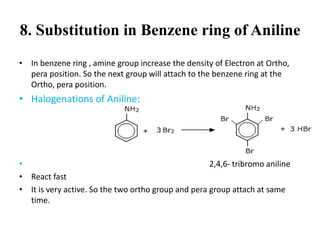
• Primary amine reaction with CHCl3 and KOH under the 60 0-70 0 degree temperature.
Then isocyanide[Carbyl-amine] with unpleasent smell found.
• Aliphatic Amine-
• Aromatic Amine-
• Secondary and tertiary amines have no reaction.](https://image.slidesharecdn.com/aminesintroclassficationnomenclaturephysicalpropertiesreactions-170722060235/85/Amines-20-320.jpg)
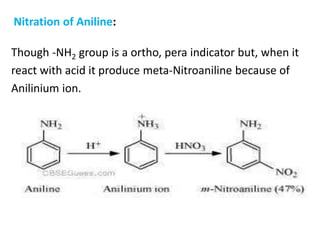
![7. Oxidation of Amines
Oxidation with KMnO4 : H
• CH3-CH2-NH2 + [O] H2SO4
KMnO4 CH3-CH=NH H+ CH3-C=O +NH3
ethylamine ethanal
Primary aliphatic amines oxidation by KMnO4 and produce ethanal.
Oxidation with H2O2 :
-NH2 + 3 H2O2 -NO2 + 4H2O
Aniline oxidation with hydrogen peroxide; produce nitro benzene and water.](https://image.slidesharecdn.com/aminesintroclassficationnomenclaturephysicalpropertiesreactions-170722060235/85/Amines-21-320.jpg)