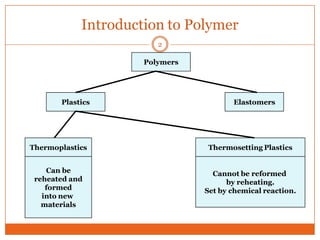
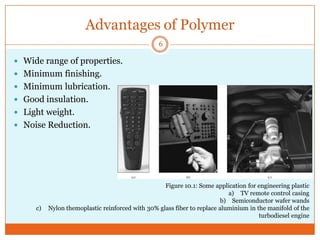
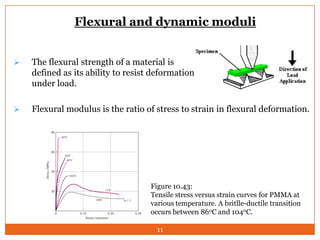
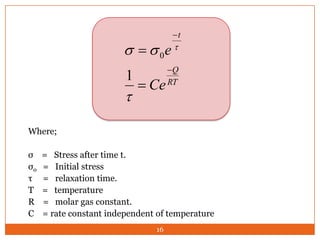
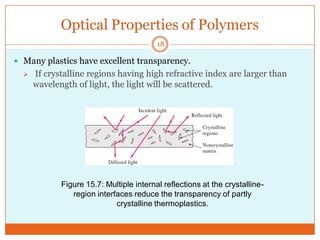
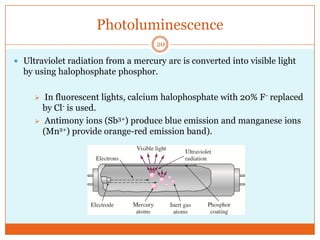
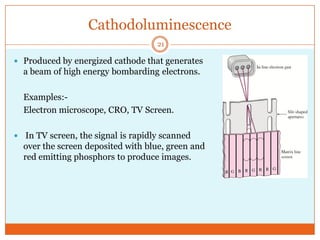
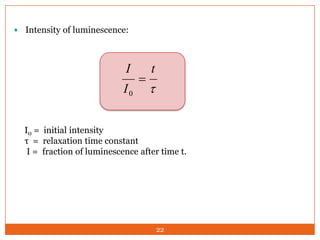
This document provides an overview of polymers including definitions of key terms like plastics, elastomers, thermoplastics, and thermosetting plastics. Thermoplastics can be remelted and reformed, while thermosetting plastics set permanently once formed. Examples of properties, mechanical behaviors like creep and stress relaxation, optical properties including luminescence, and polymerization processes are also summarized. The document concludes with references for further reading on materials science topics.