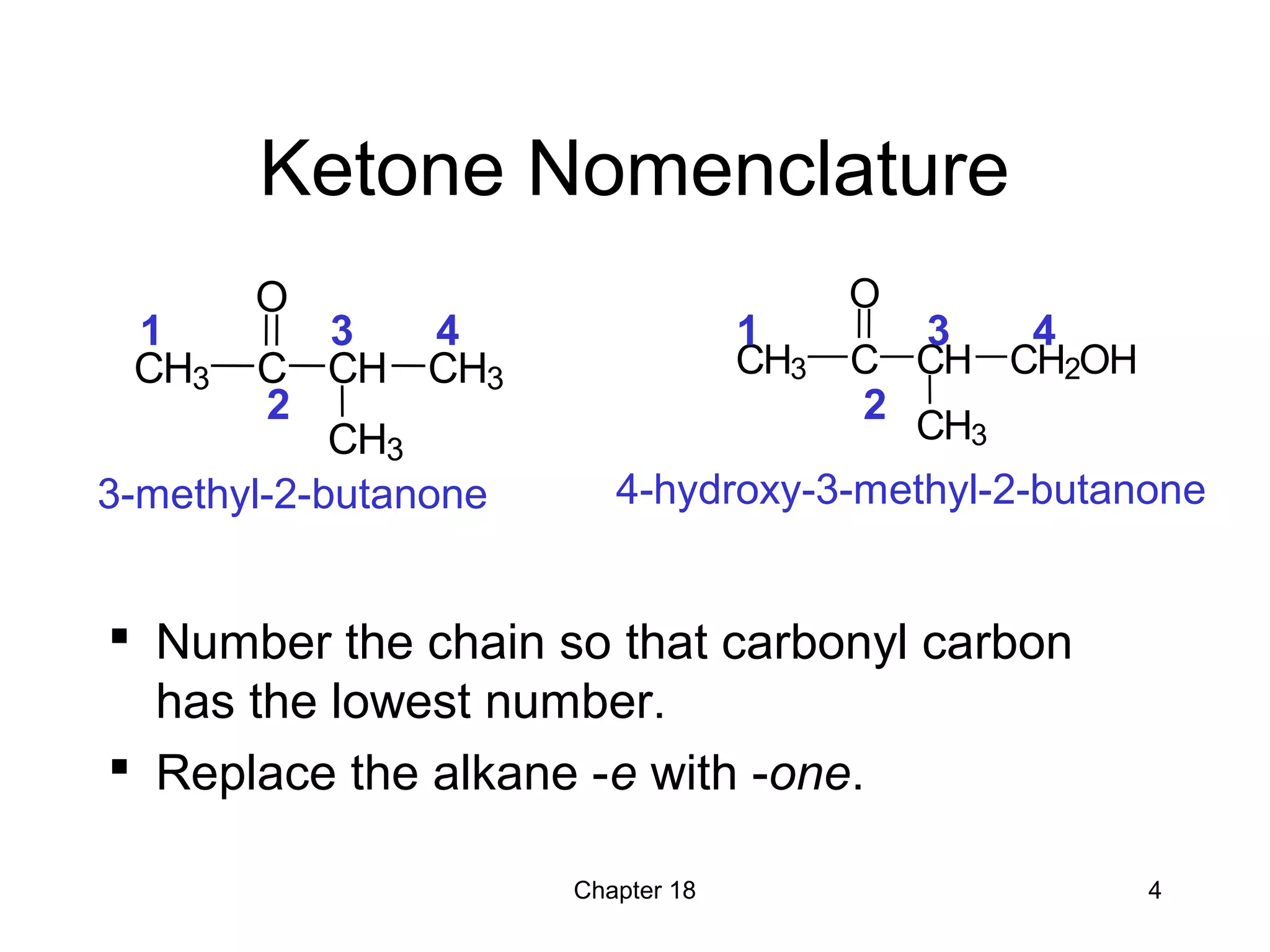
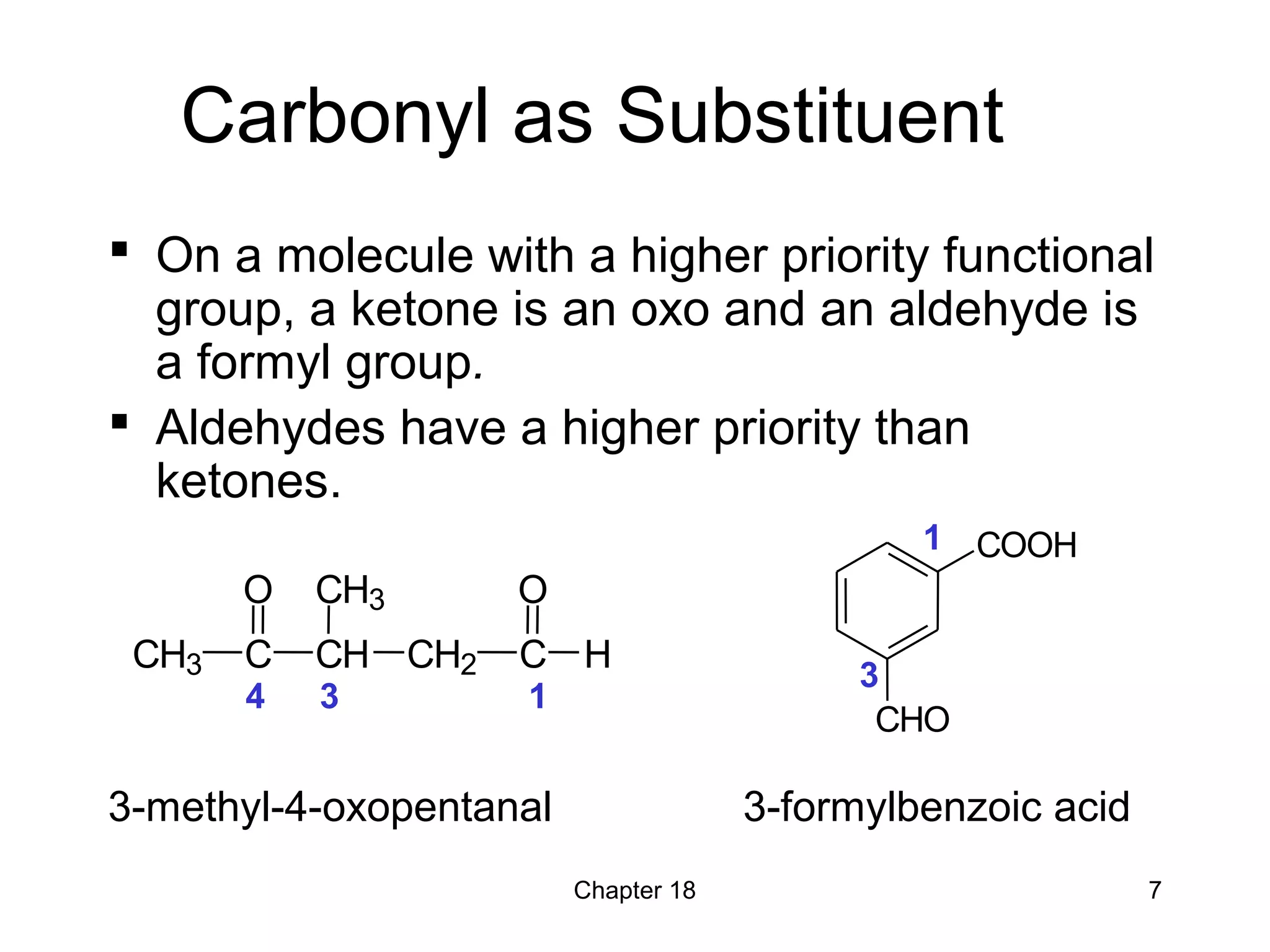
1) The document summarizes key concepts about ketones and aldehydes from an organic chemistry textbook chapter, including their structures, nomenclature, physical properties, reactions, and industrial uses.
2) Methods of synthesizing ketones and aldehydes are discussed, including oxidation of alcohols, Friedel-Crafts acylation, and reactions of nitriles, acid chlorides, and carboxylic acids.
3) Common reactions of ketones and aldehydes described include nucleophilic addition, hydration, imine and acetal formation, reductions, and oxidations.