Embed presentation
Downloaded 39 times

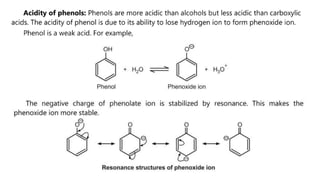
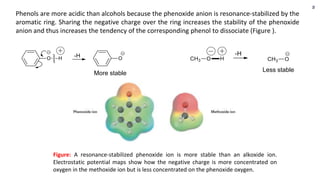
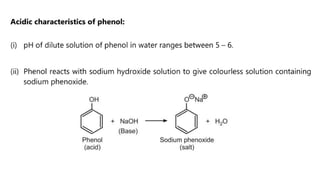
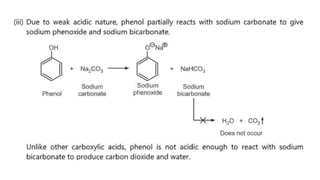
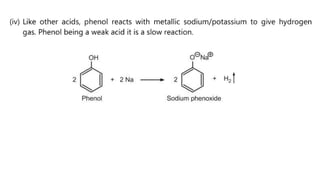
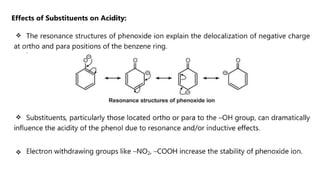
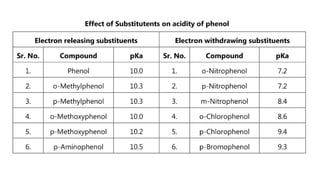
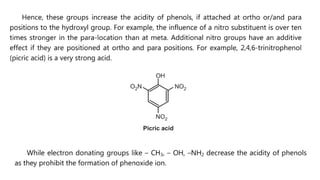
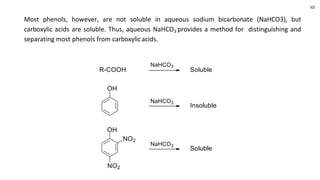
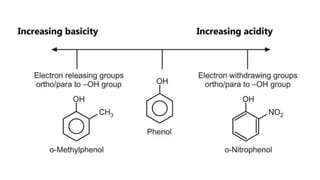


Phenols are more acidic than alcohols due to the resonance stabilization of the phenoxide anion by the aromatic ring, which increases its stability and propensity to dissociate. Most phenols are not soluble in aqueous sodium bicarbonate, unlike carboxylic acids, allowing for their separation. A video link is provided for further information.











