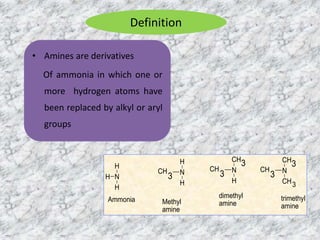
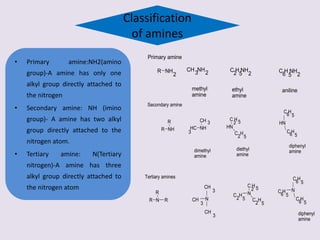
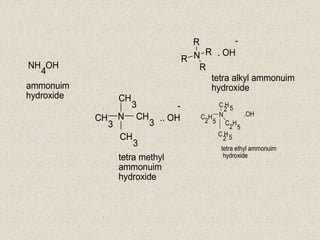
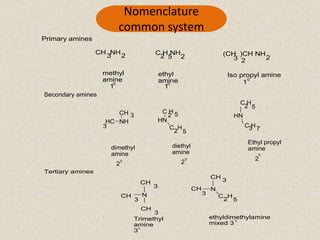
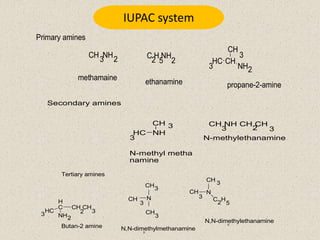
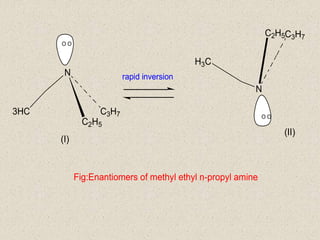
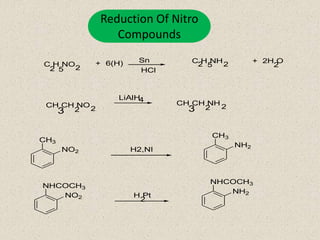
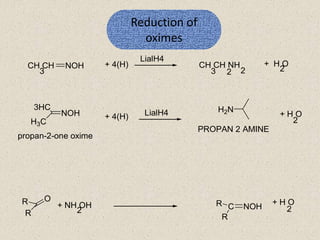
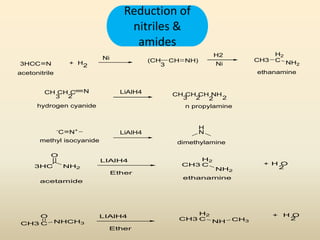
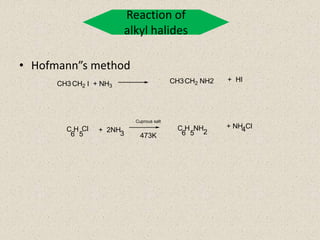
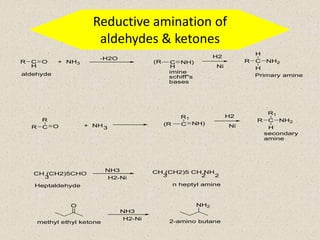
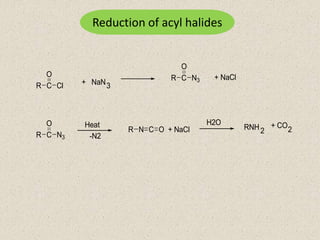
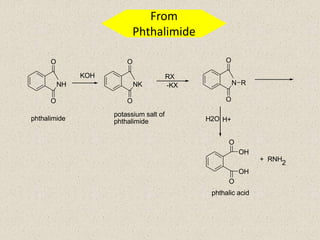
The document provides an overview of aliphatic amines, defining them as ammonia derivatives where hydrogen atoms are replaced by alkyl or aryl groups. It classifies amines into primary, secondary, and tertiary types based on the number of alkyl groups attached to the nitrogen atom and discusses nomenclature and methods of preparation through various reduction reactions. Additionally, it includes examples of each category, demonstrating the chemical structure and naming conventions.