Embed presentation
Downloaded 39 times

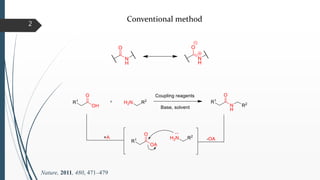
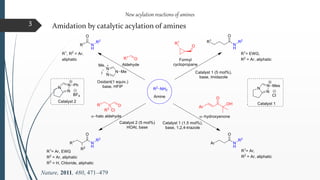




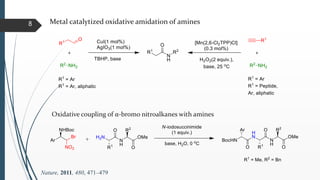



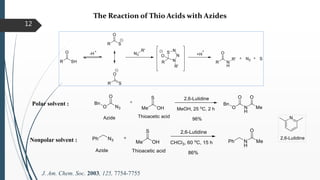
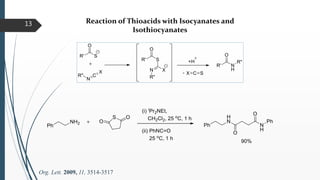



This document discusses various conventional and new methods for amidation or amide bond formation, including: 1) Catalytic acylation of amines using a-hydroxyenones or activated carboxylates. 2) Nucleophilic carbene and HOAt catalyzed amide coupling using a Breslow intermediate. 3) Milstein's catalyst for converting alcohols to amides. 4) Pd-catalyzed aminocarbonylation of aryl bromides. 5) Amidation of thioacids with azides, isocyanates, or isothiocyanates. 6) Microwave assisted amidation by acylation with amine derivatives.














