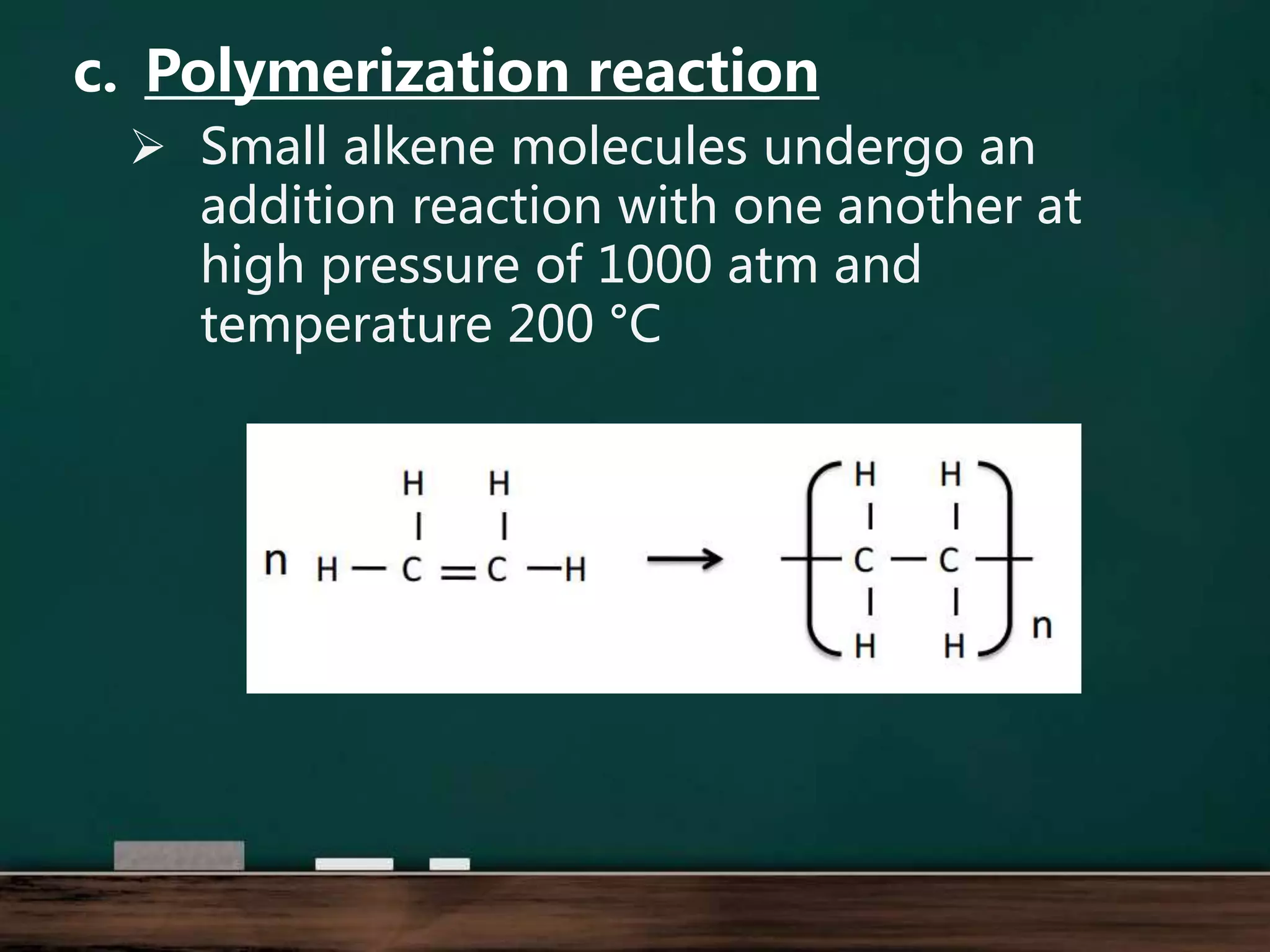
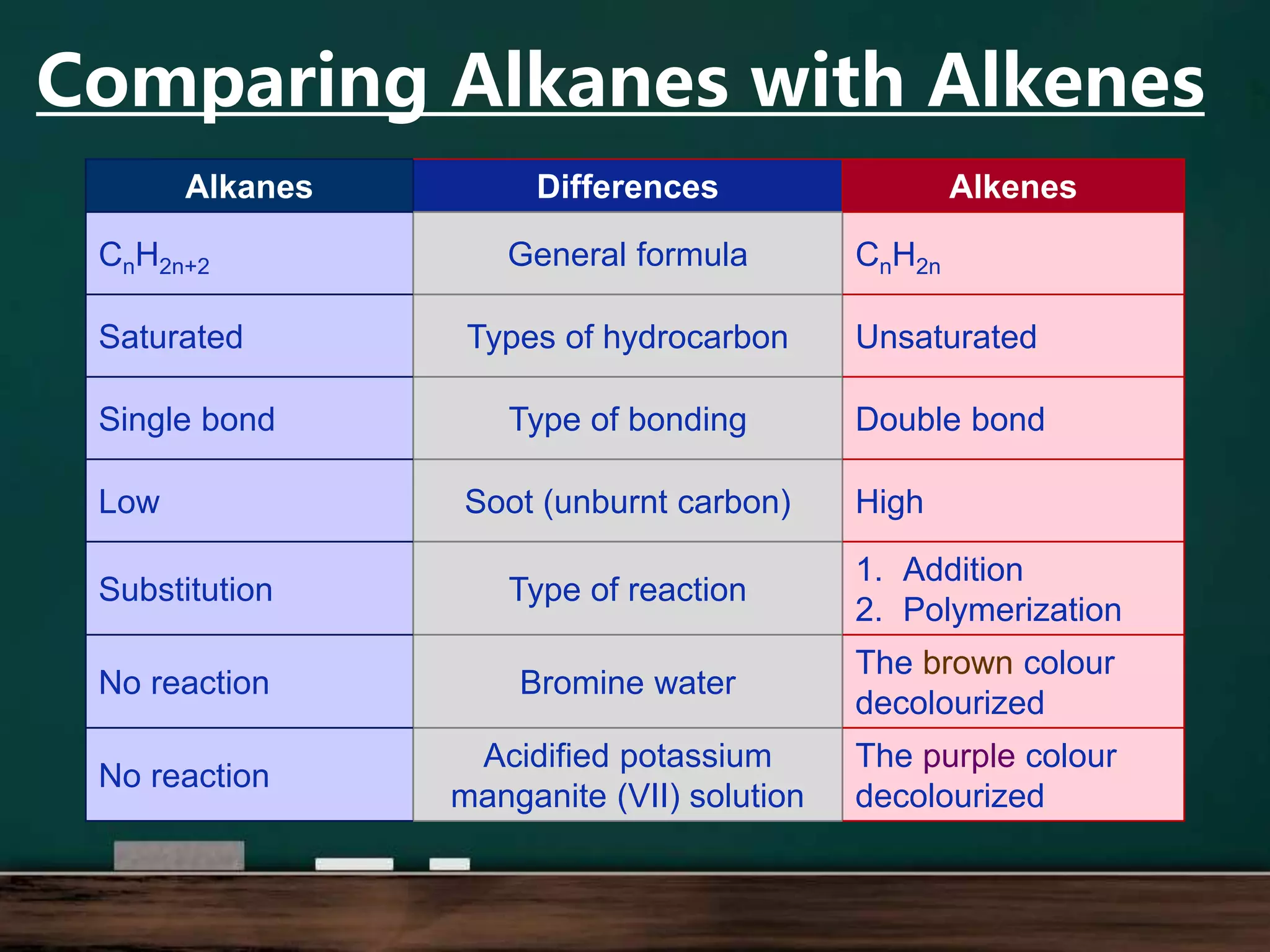
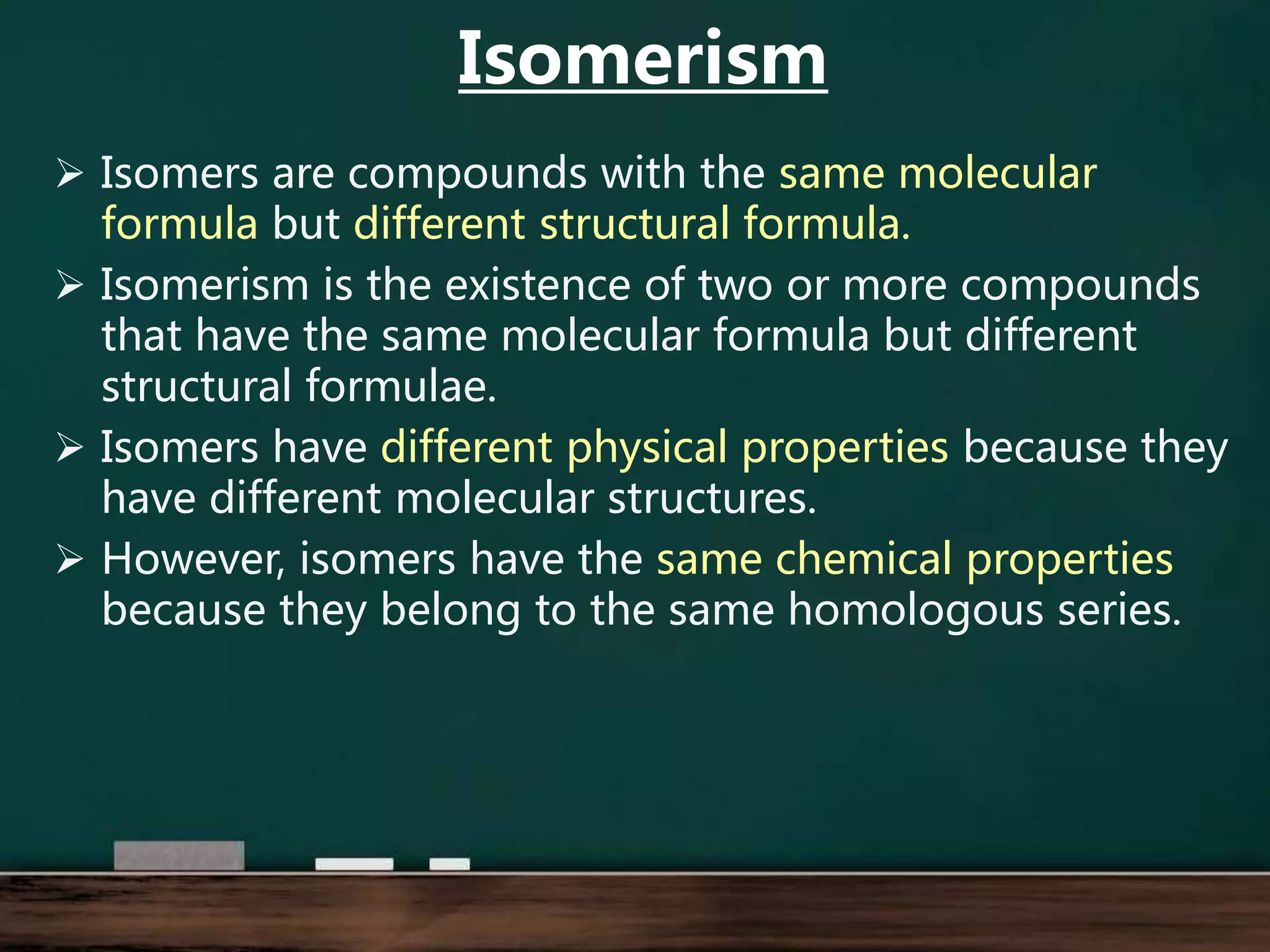
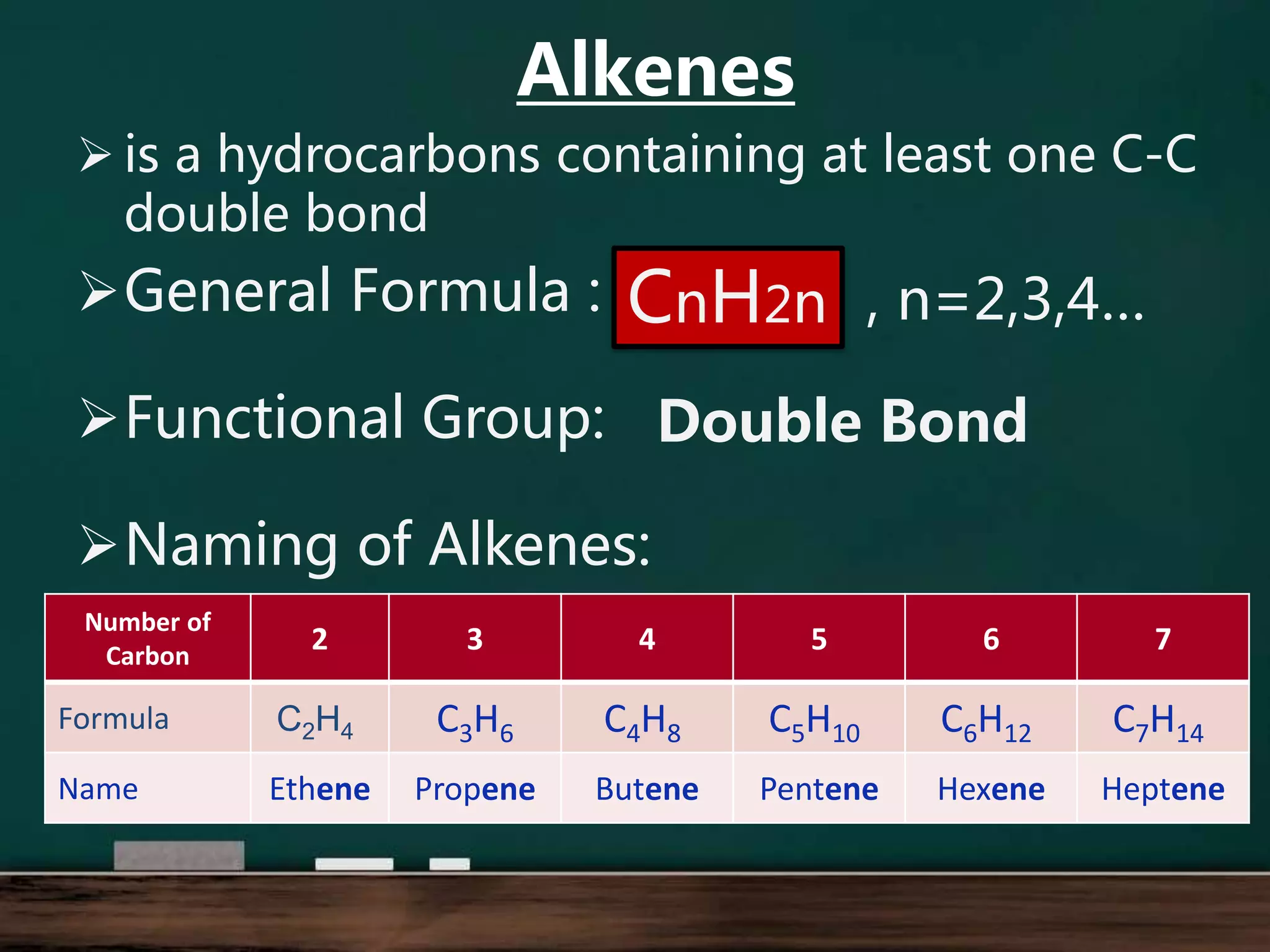
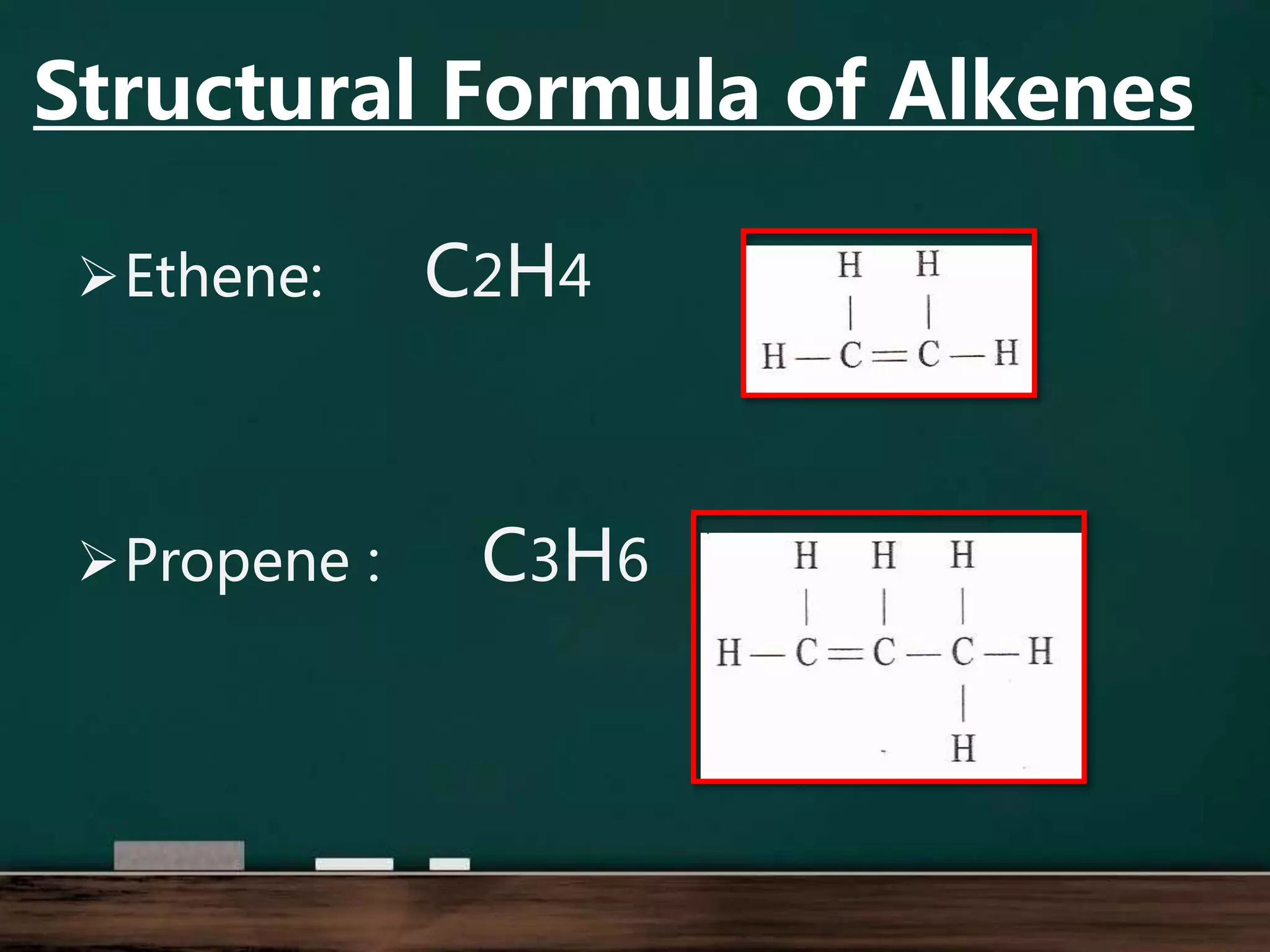
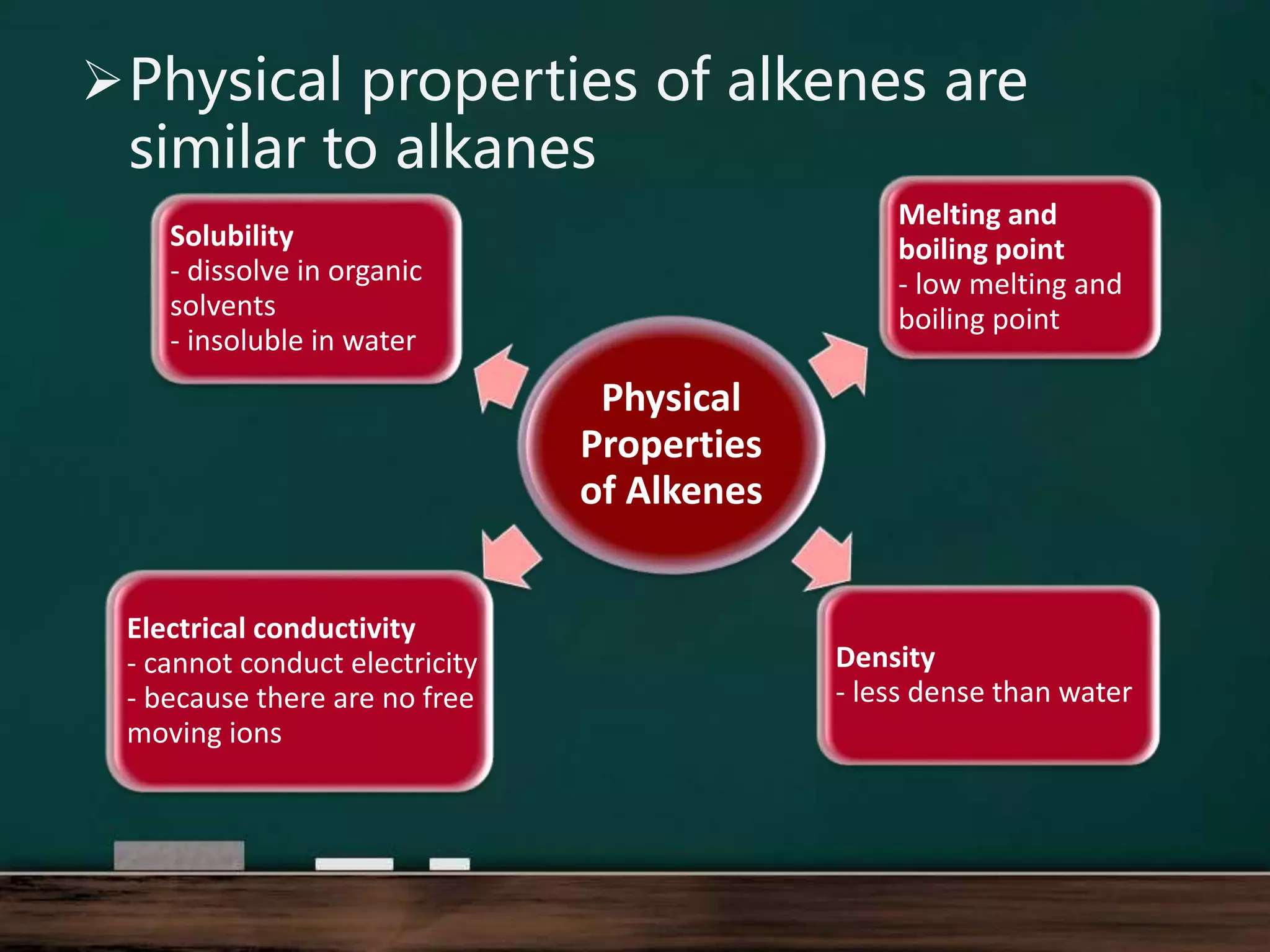
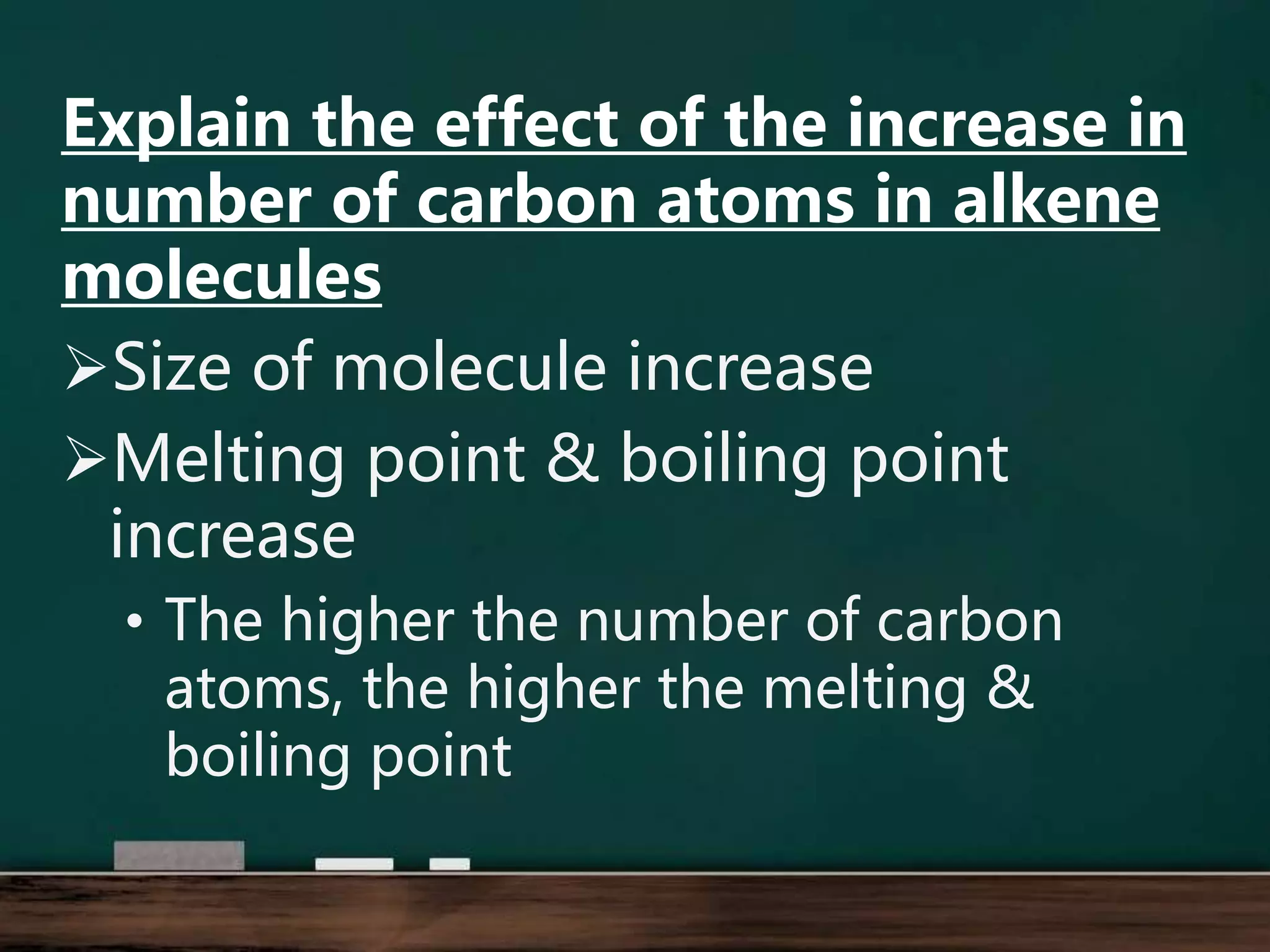
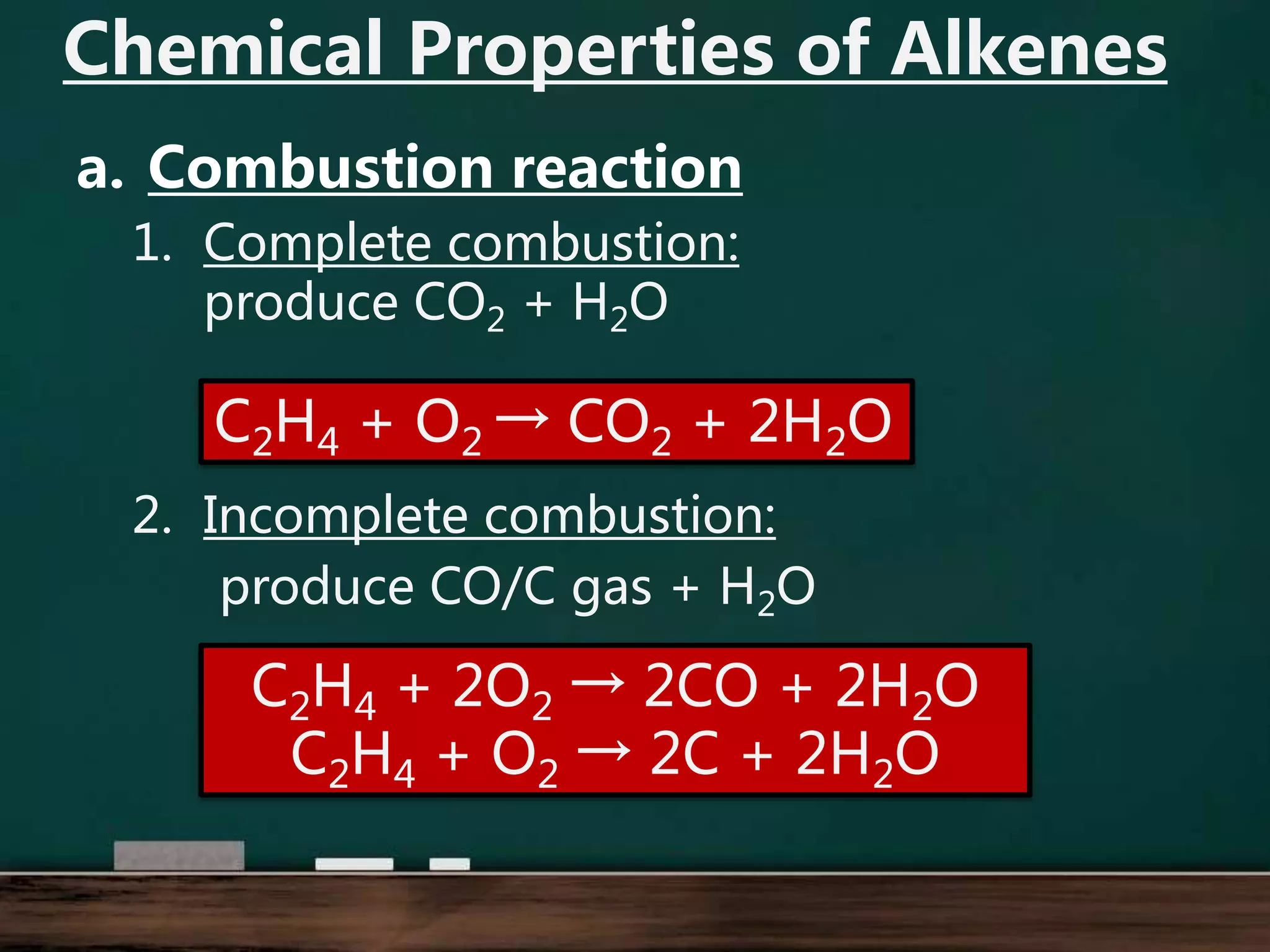
Alkenes are hydrocarbons containing at least one carbon-carbon double bond. They have lower melting and boiling points than alkanes due to weaker intermolecular forces. The number of carbons determines an alkene's name and formula. Alkenes undergo addition reactions, combustion reactions, polymerization reactions, and can be used to test for double bonds. They differ from alkanes in bonding, reactivity and ability to cause soot during combustion. Isomers are compounds with the same molecular formula but different structural formulas, resulting in different physical but same chemical properties.



![4. Addition of hydroxyl group
Alkenes react with acidified potassium manganate(VII),
KMnO4 to produce diol compound
C2H4 + [O] + H2O → C2H4 (OH)2
or
Used to test for the presence of a C-C double bond](https://image.slidesharecdn.com/alkane-170317044809/75/Alkene-9-2048.jpg)