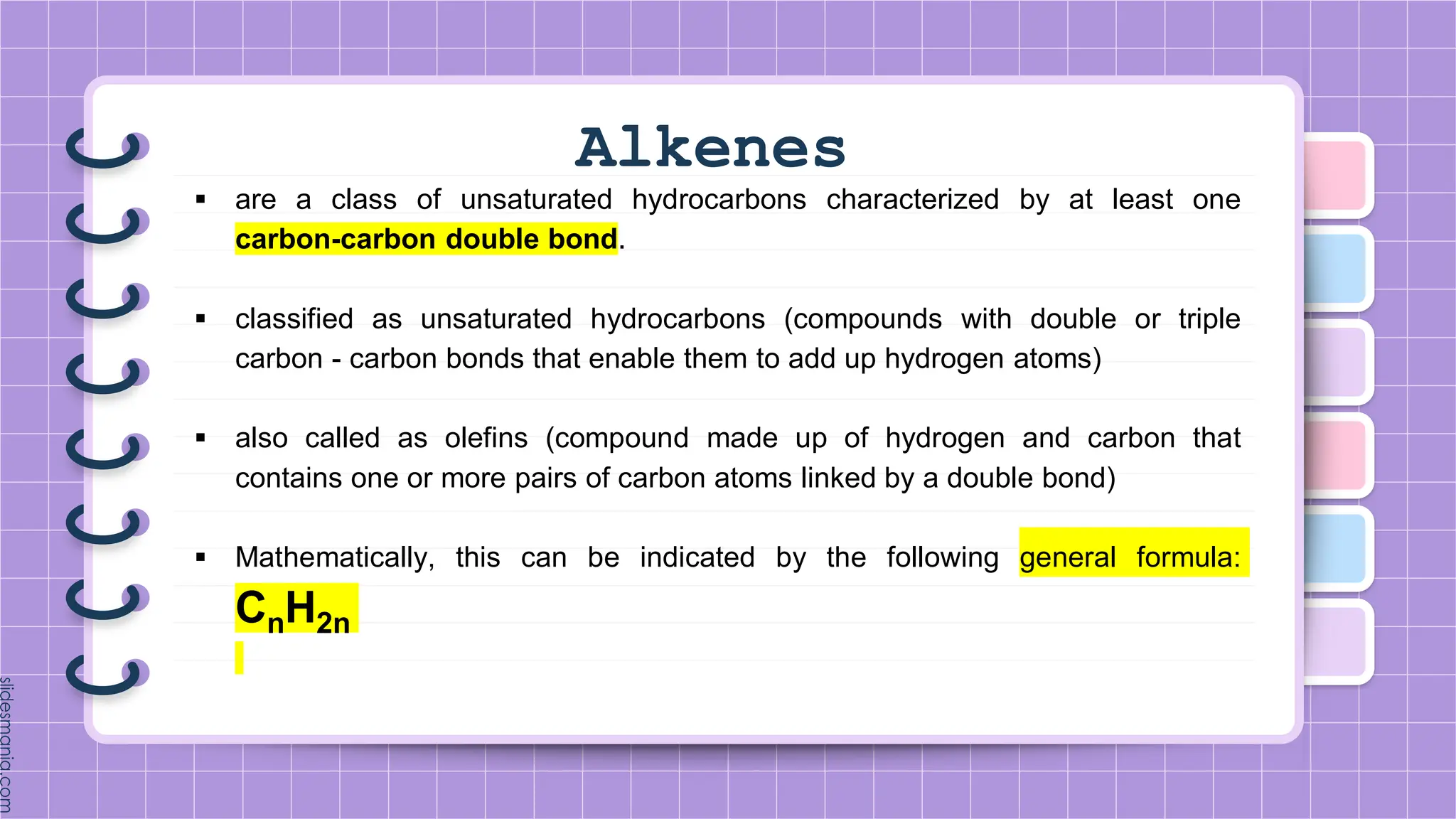
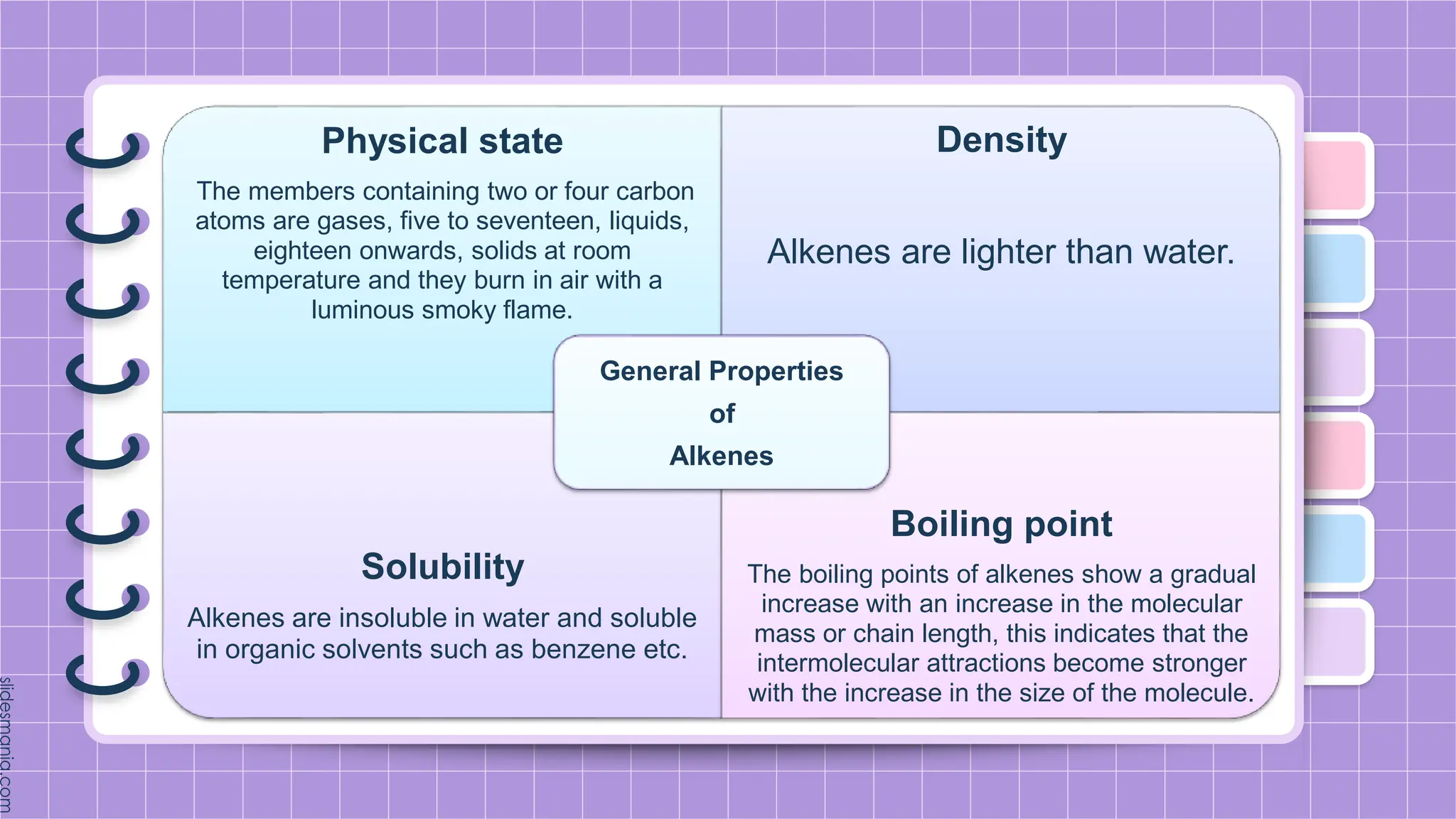
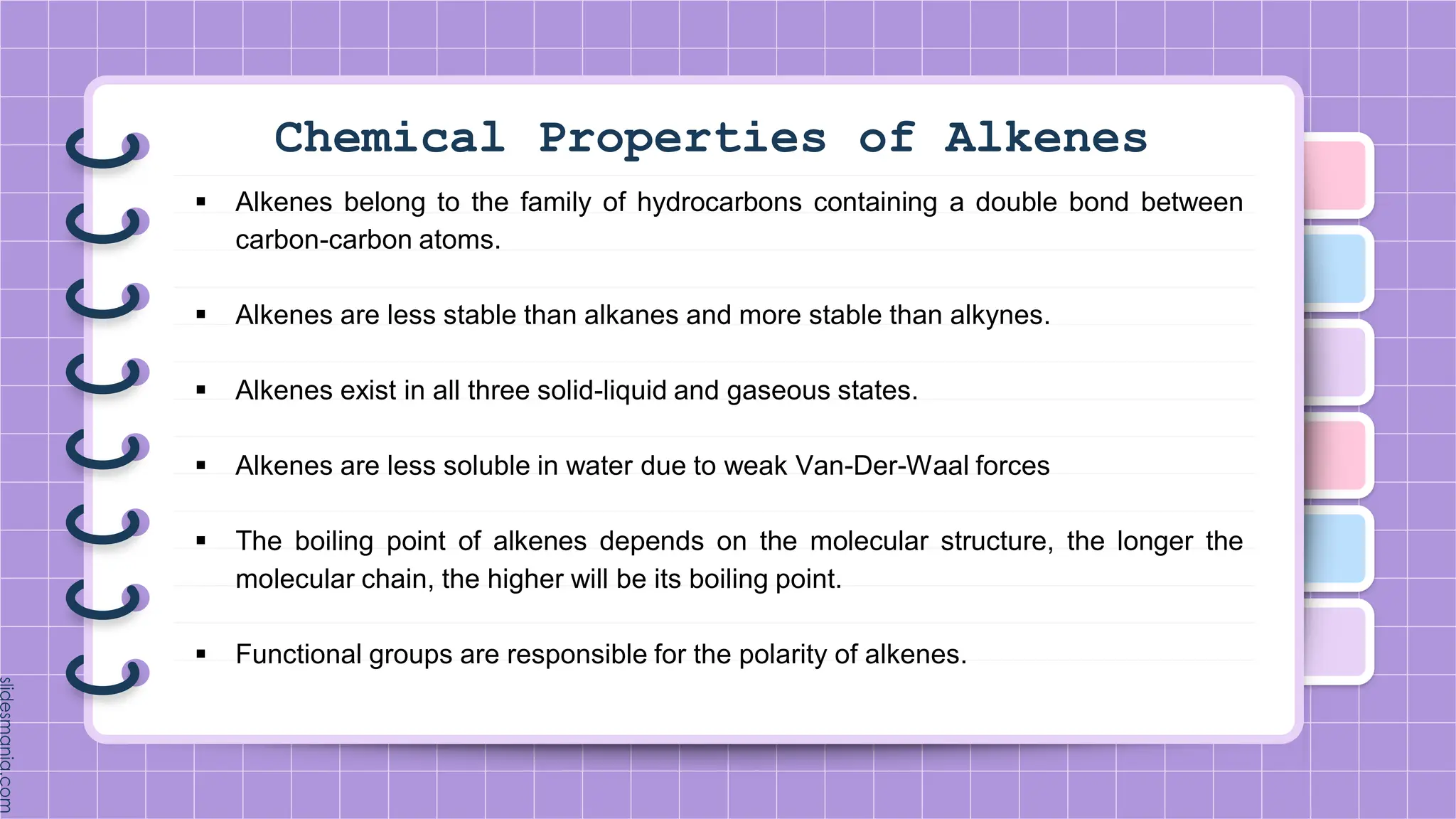
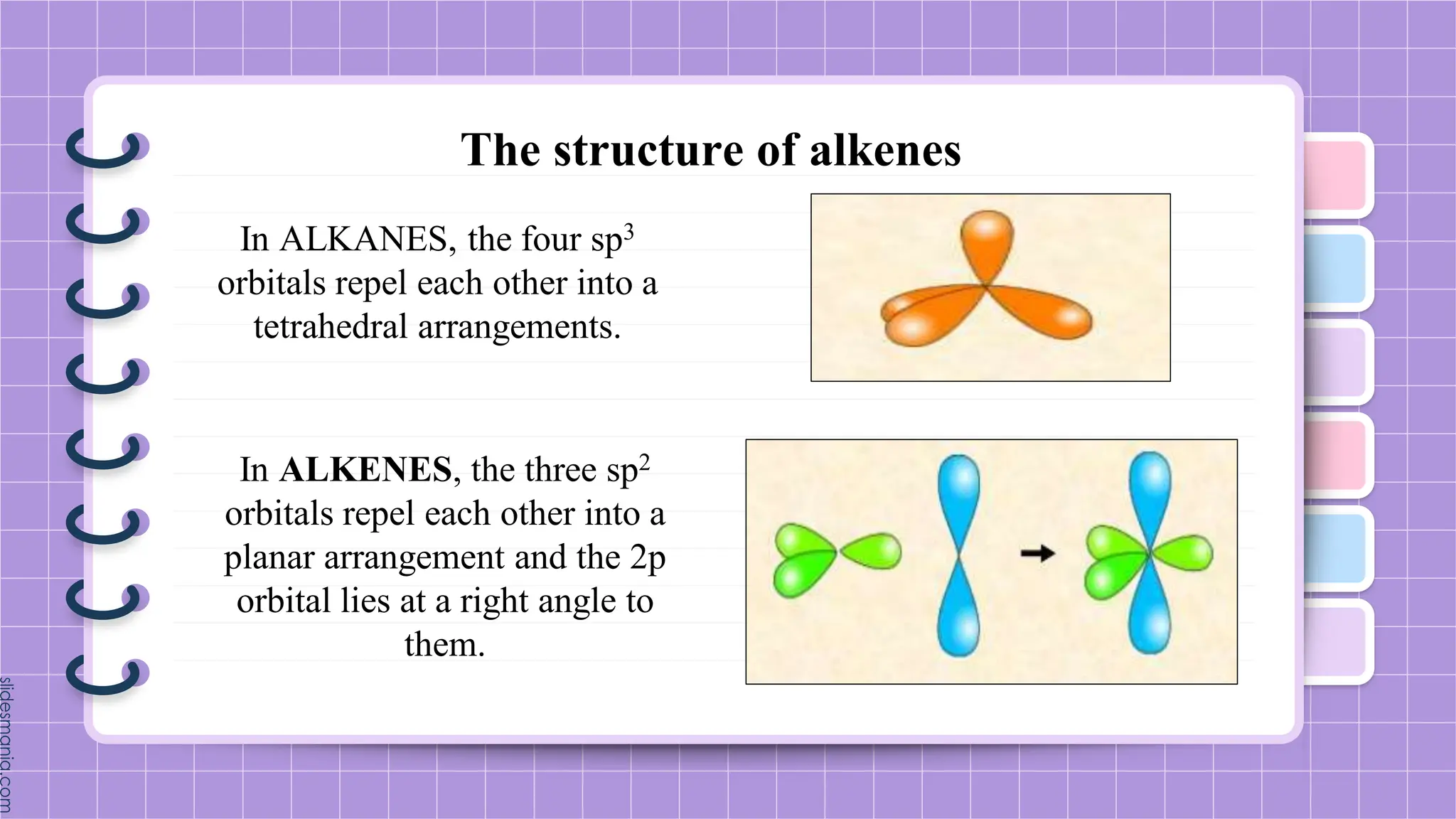
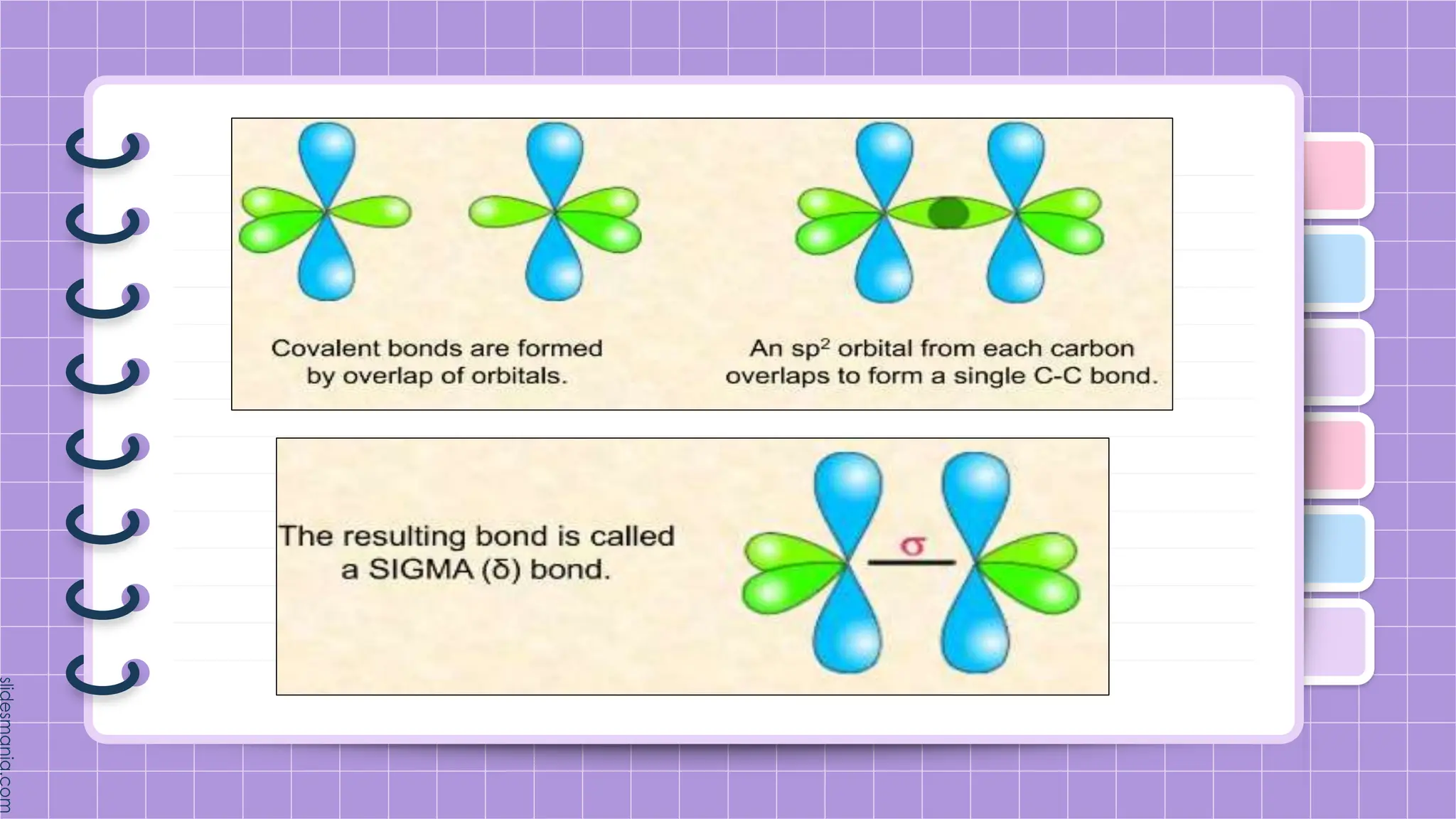
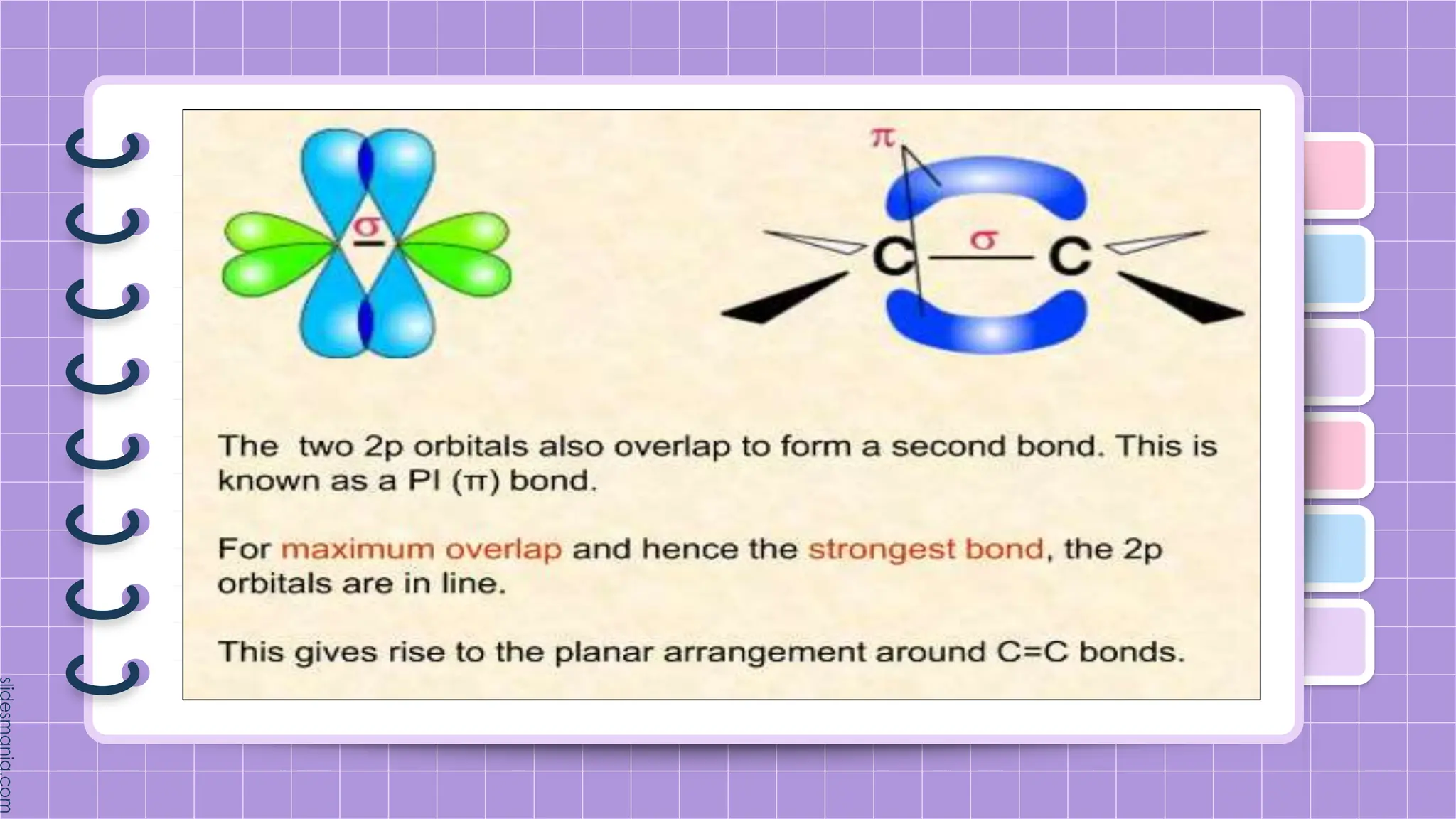
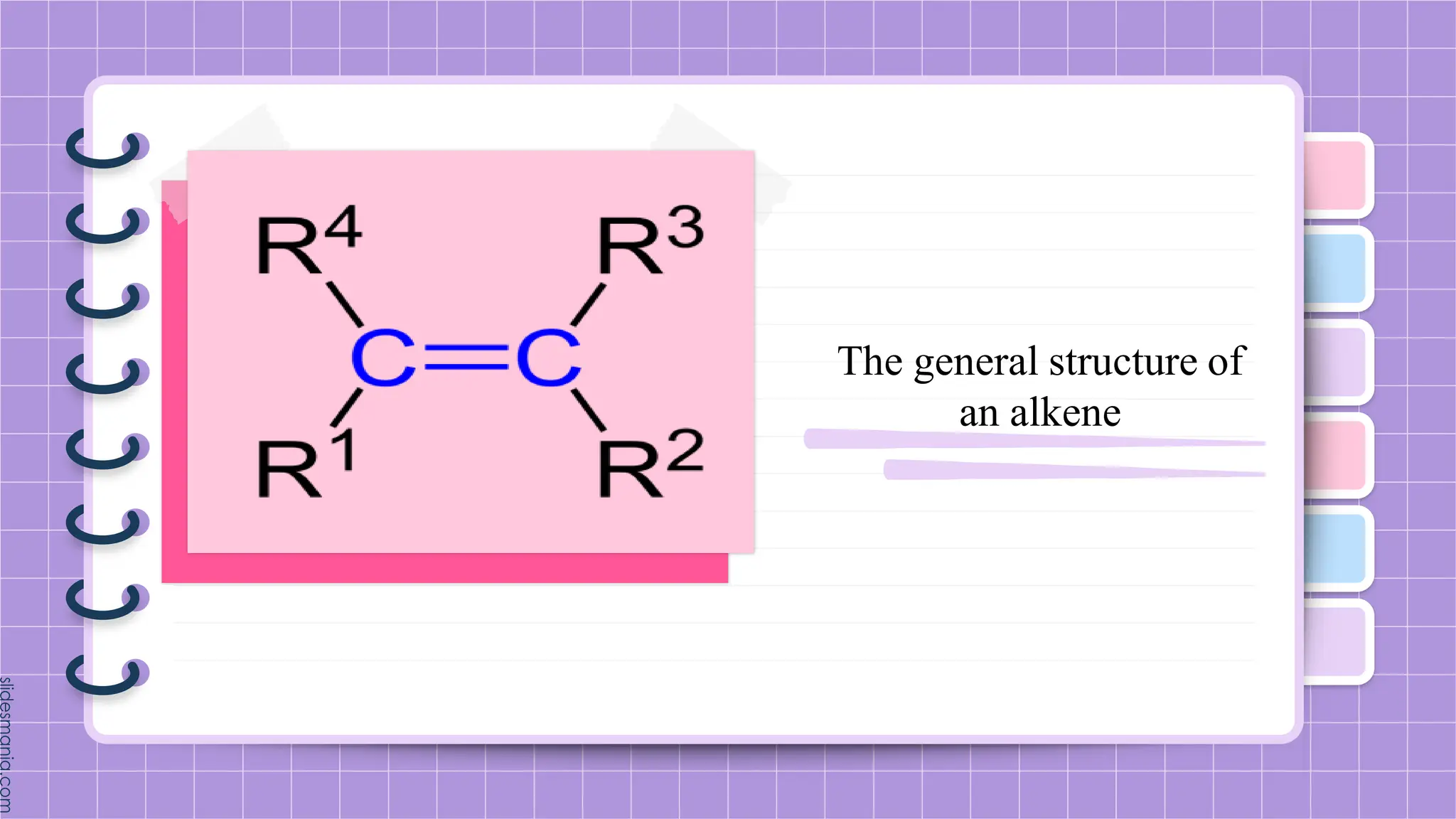
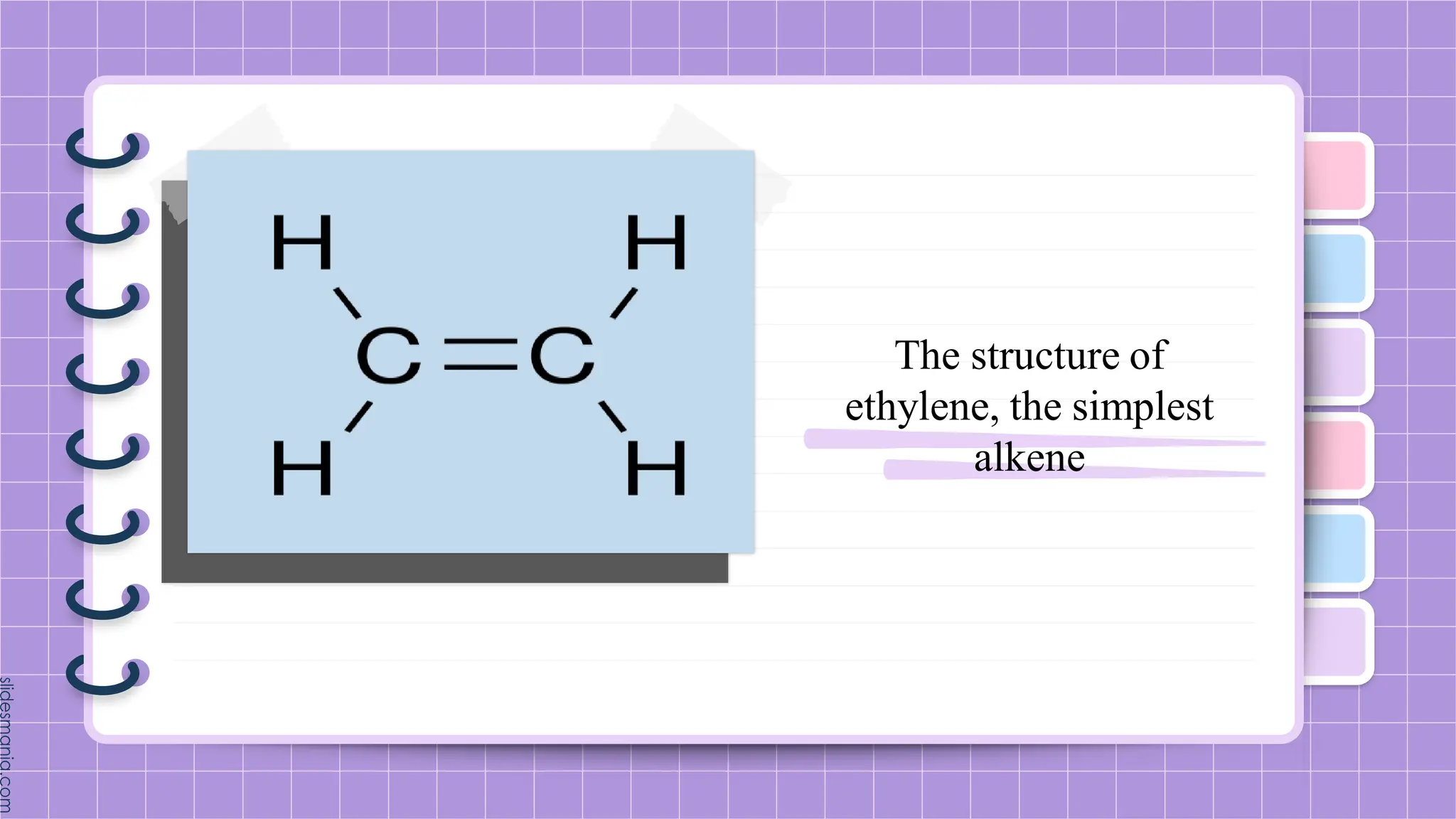
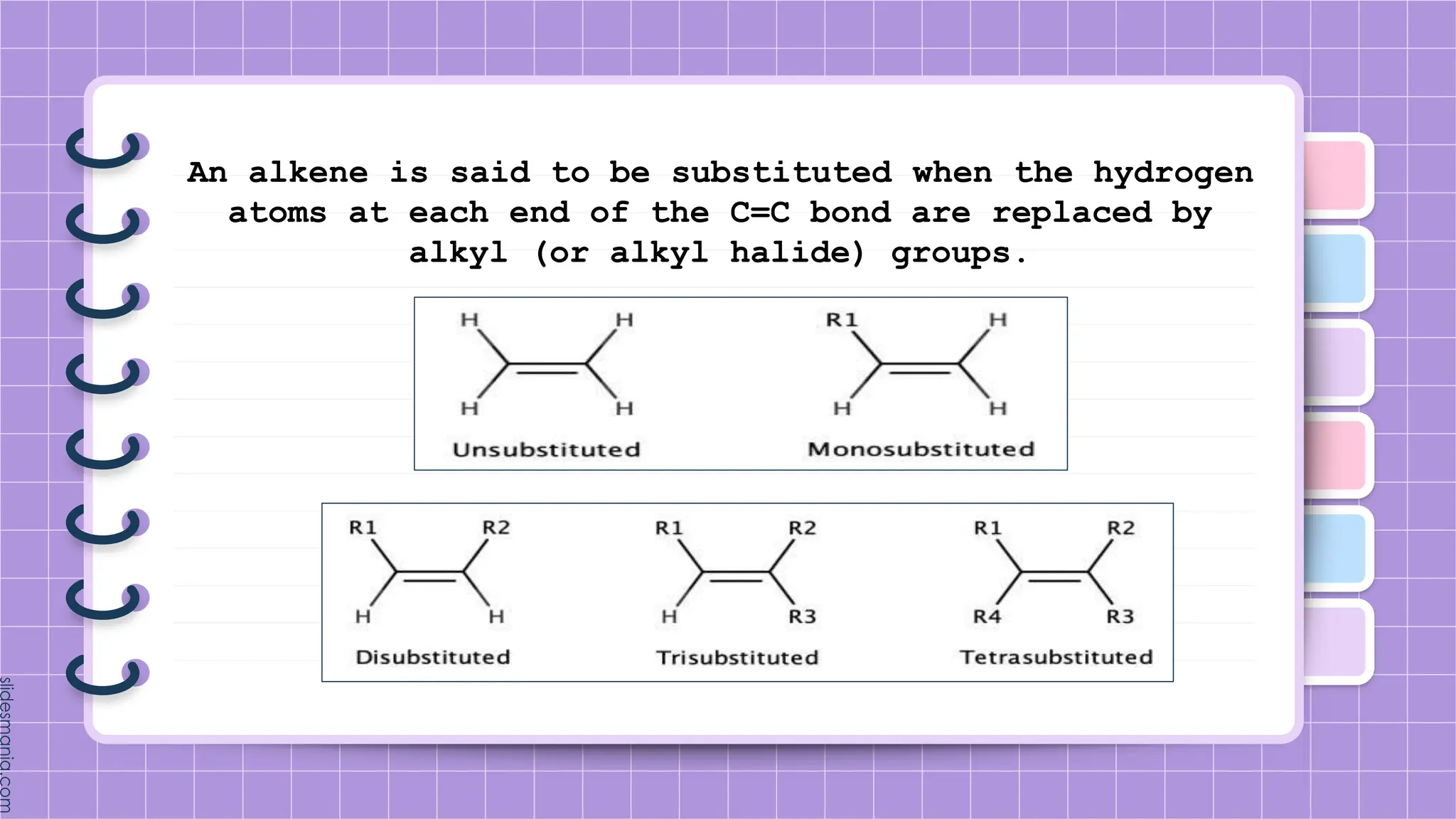
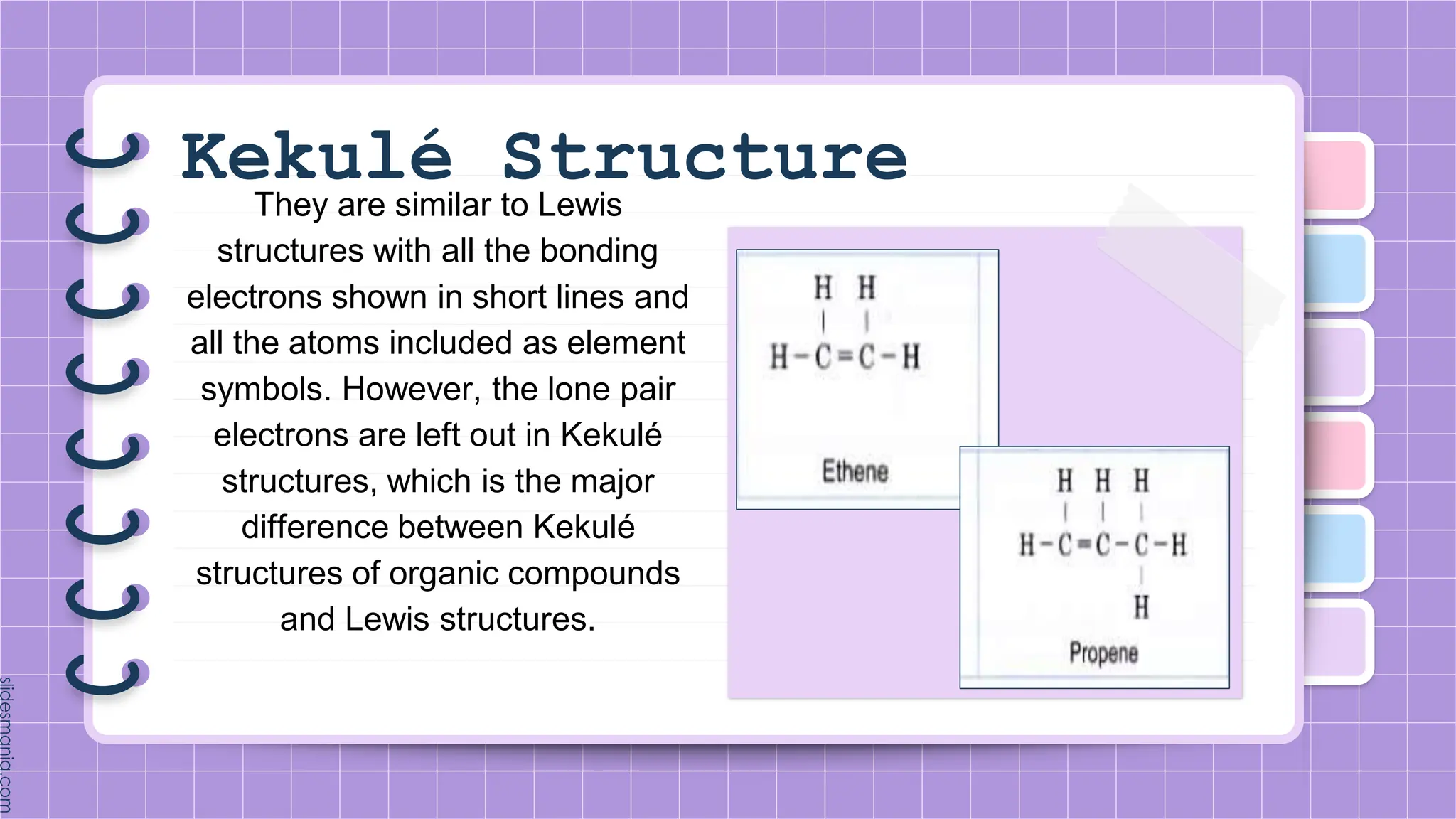
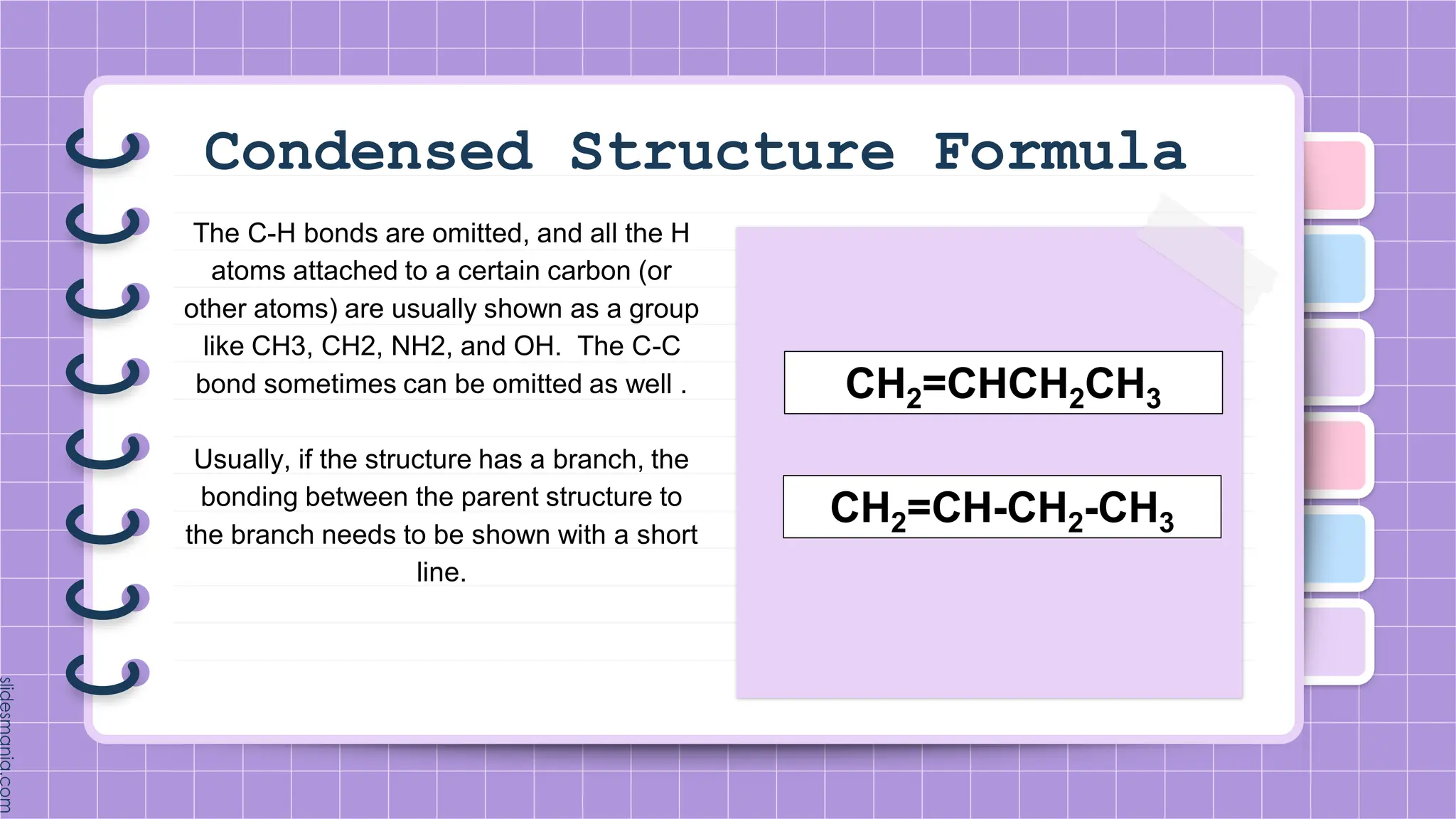
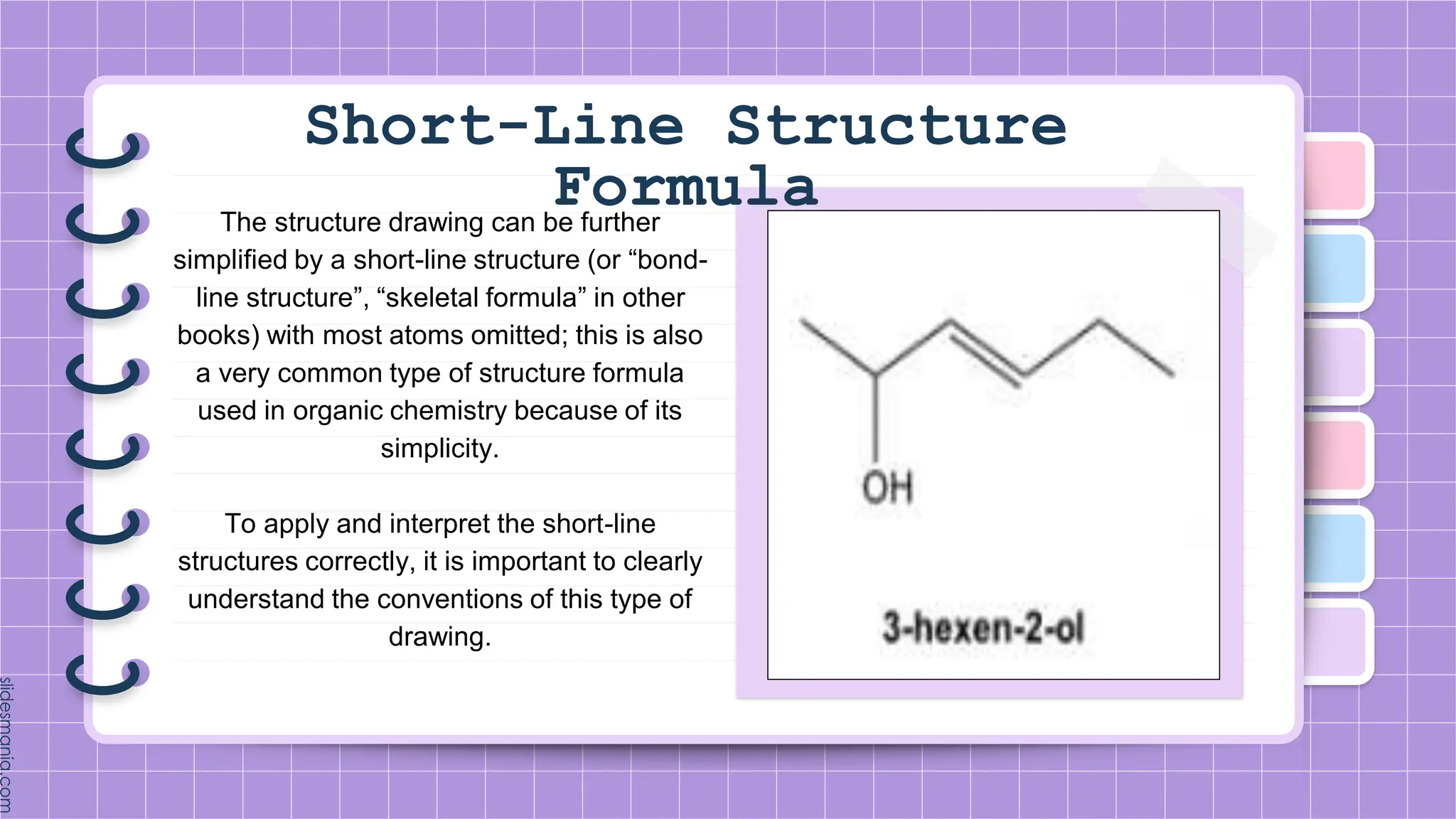
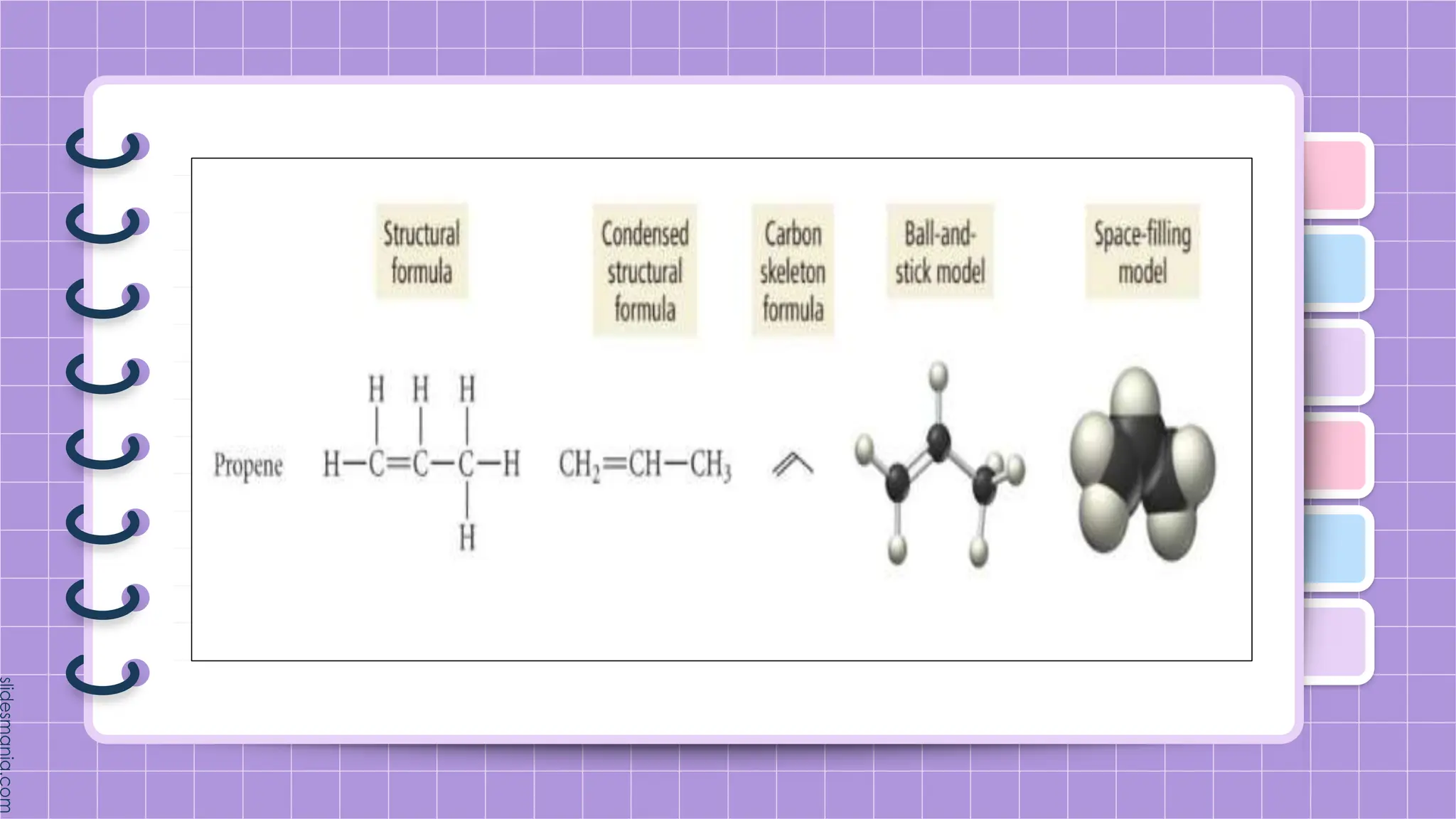
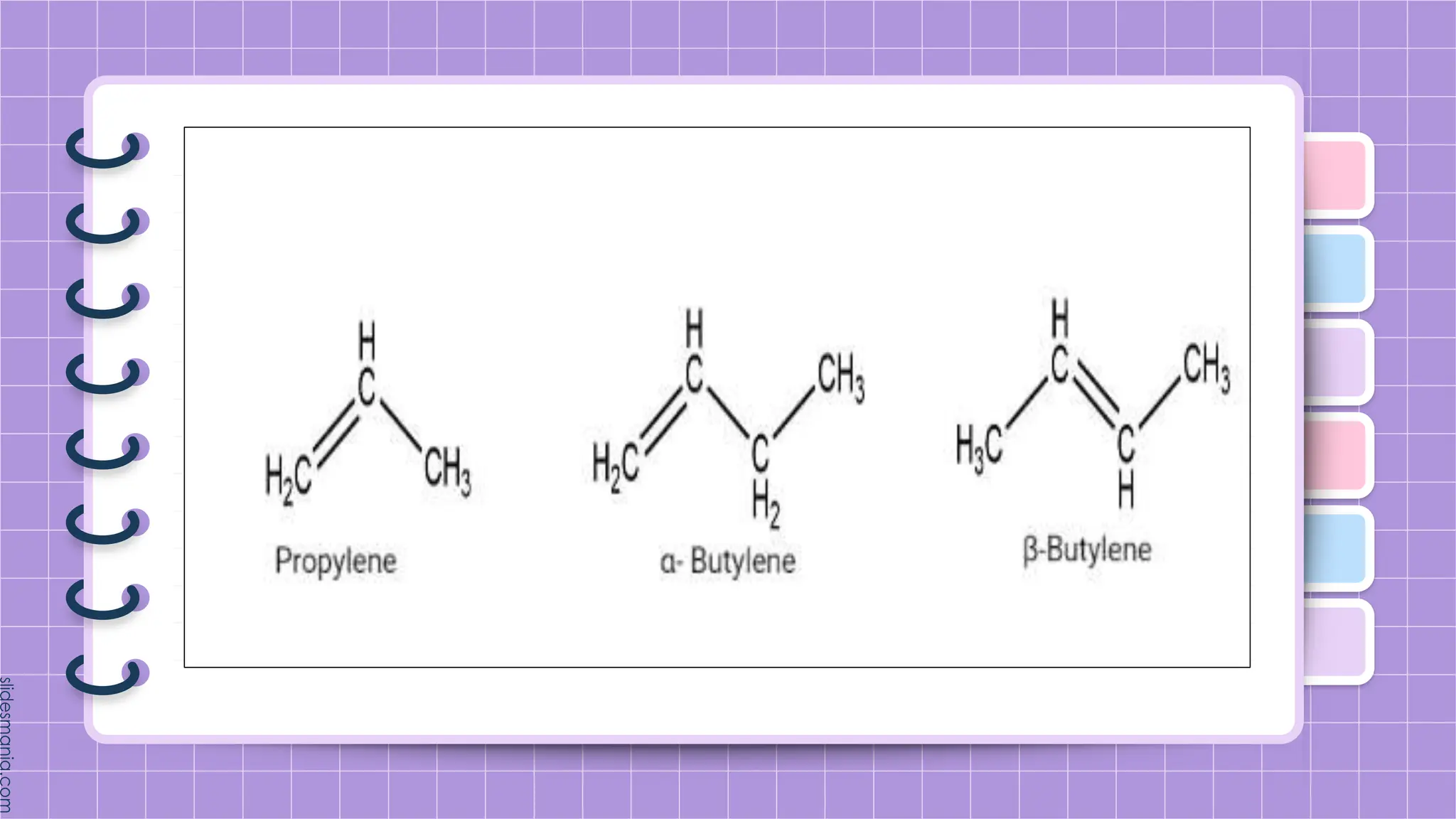
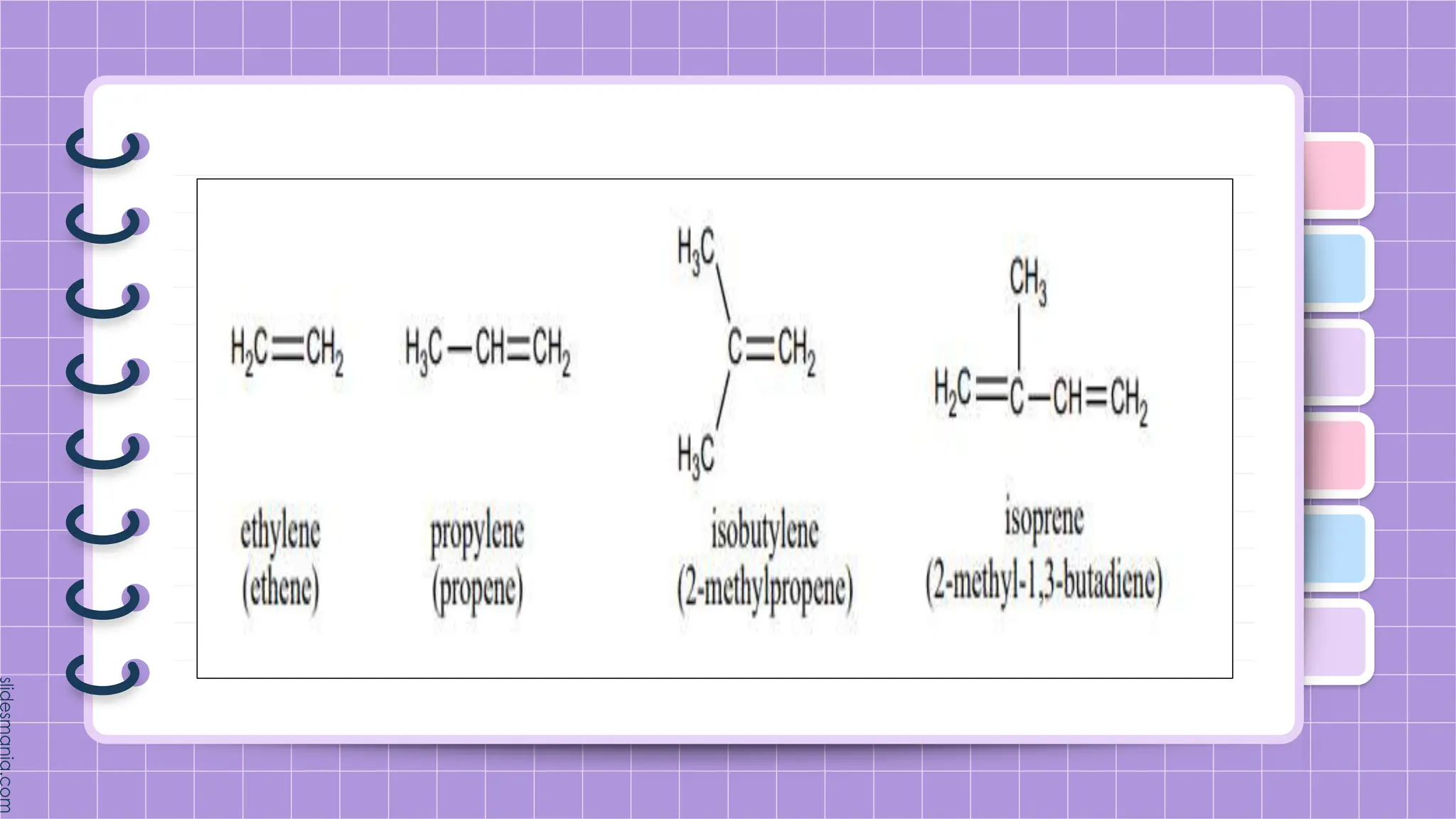
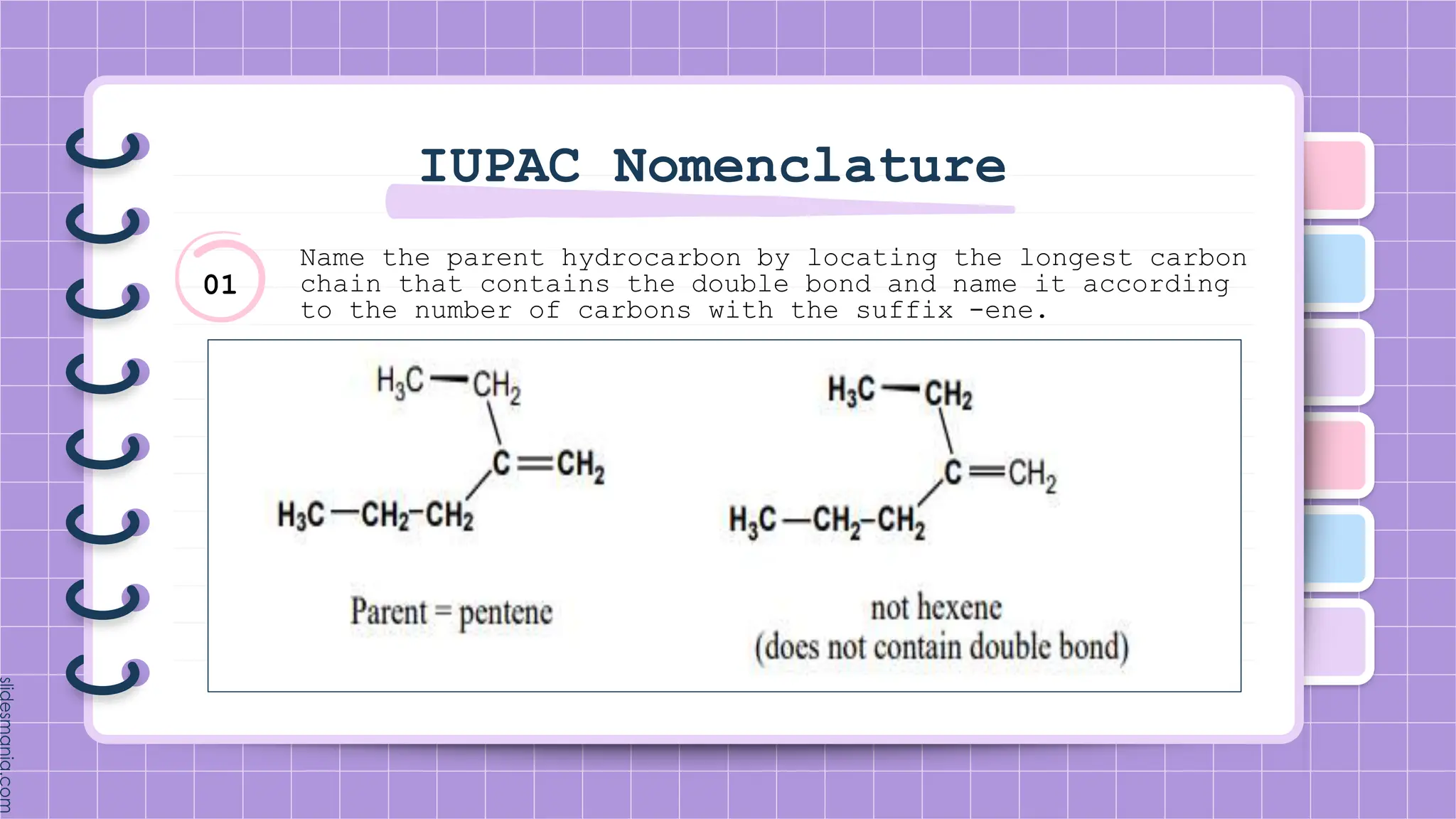
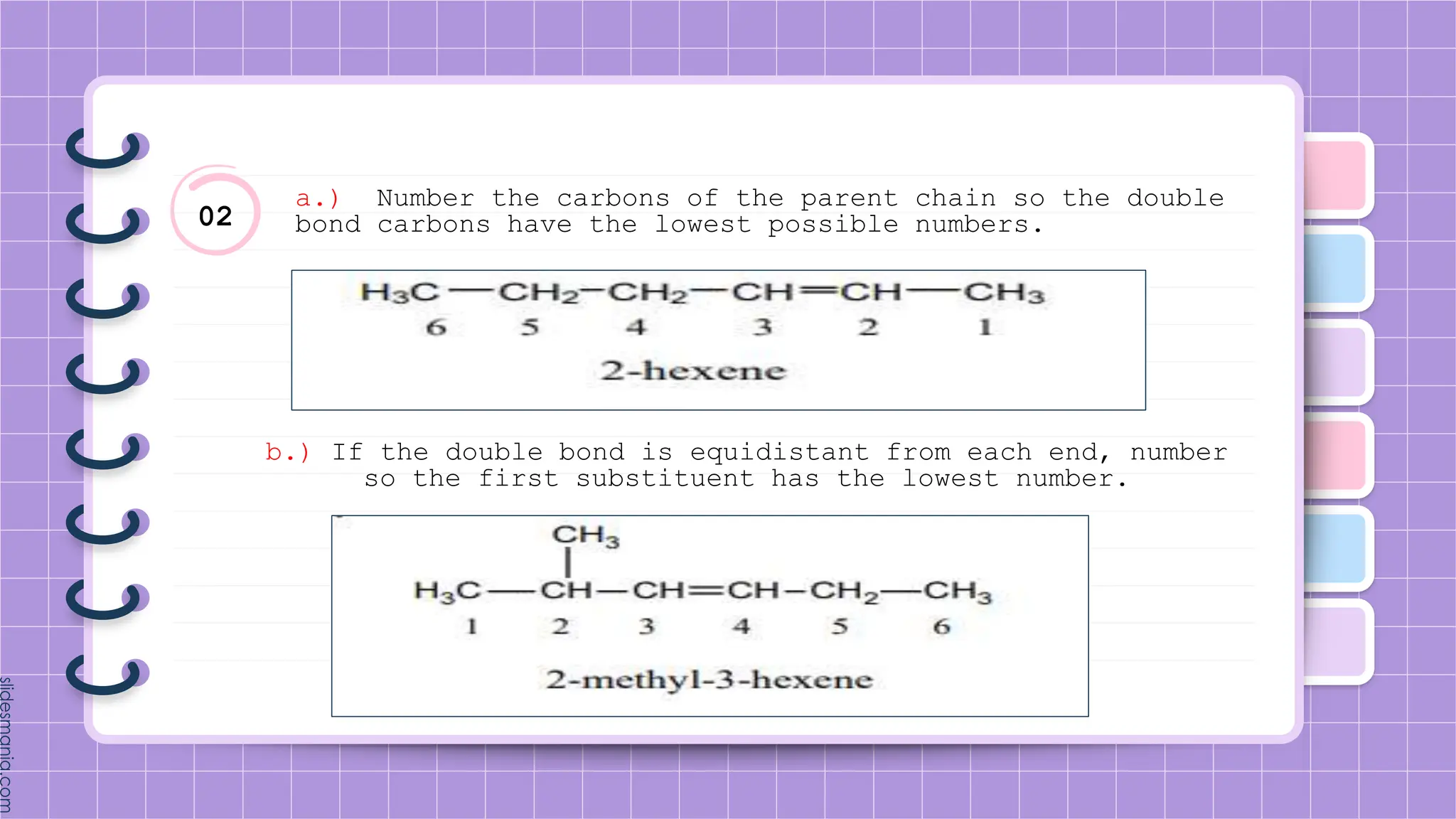
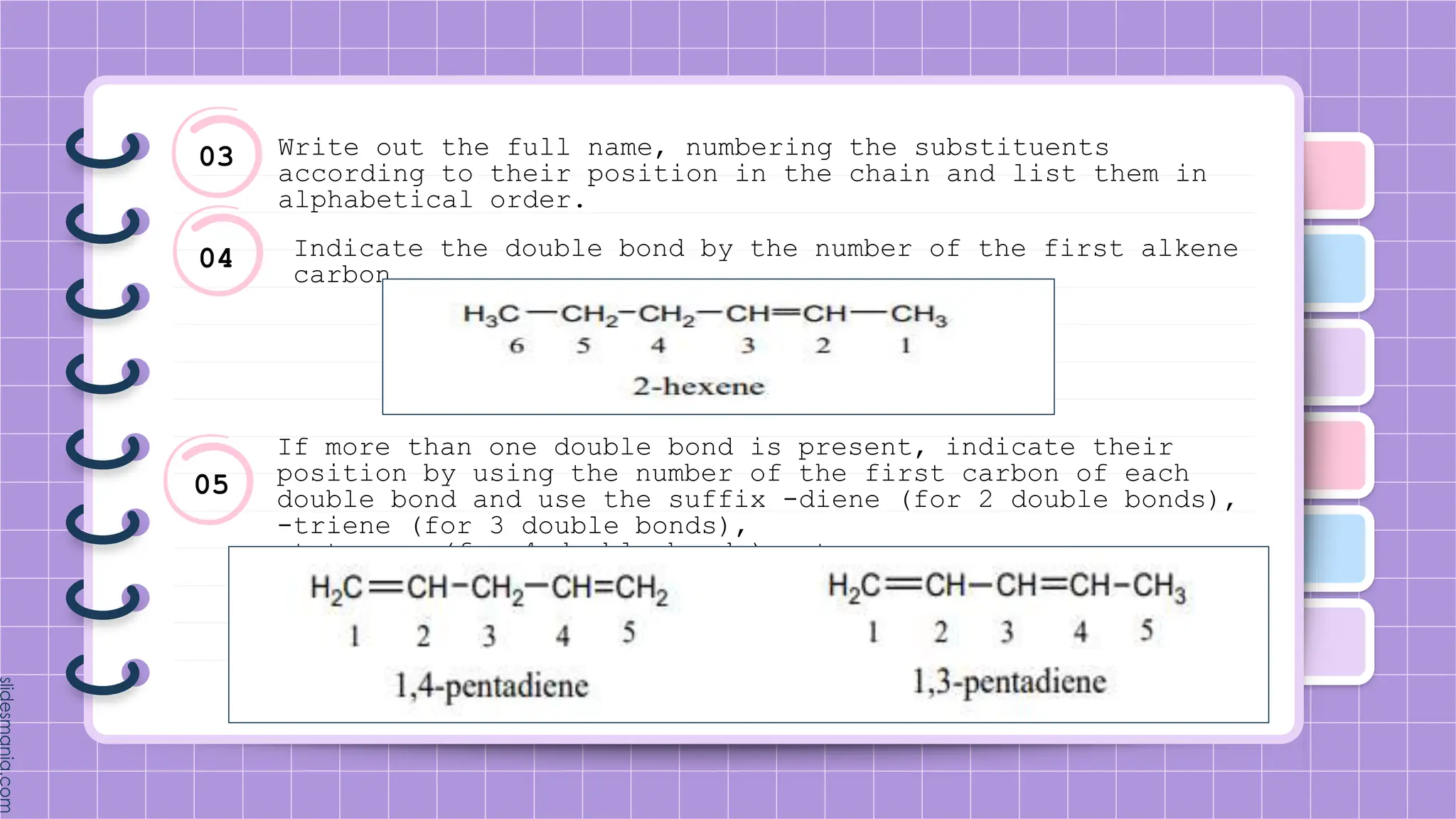
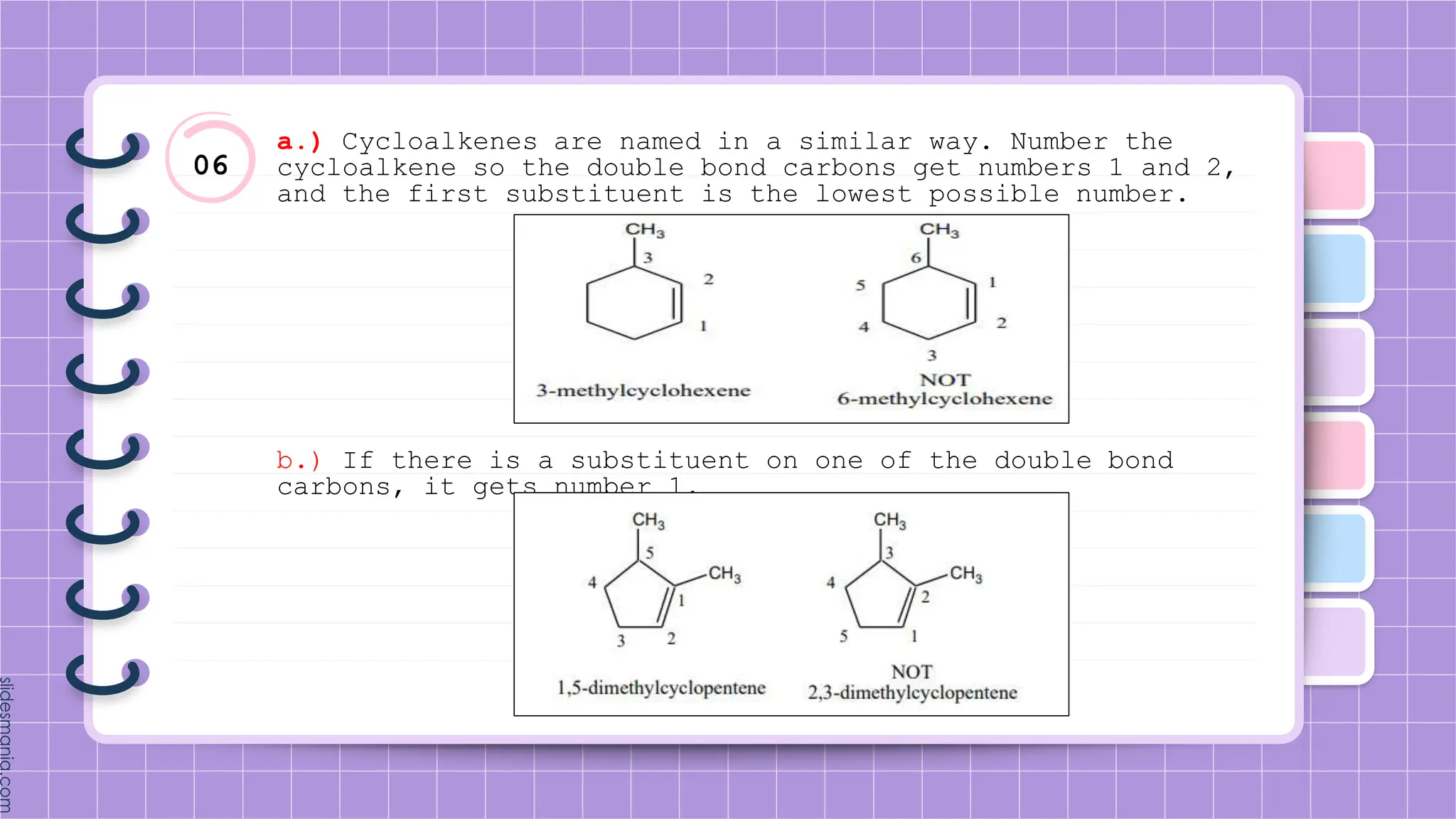
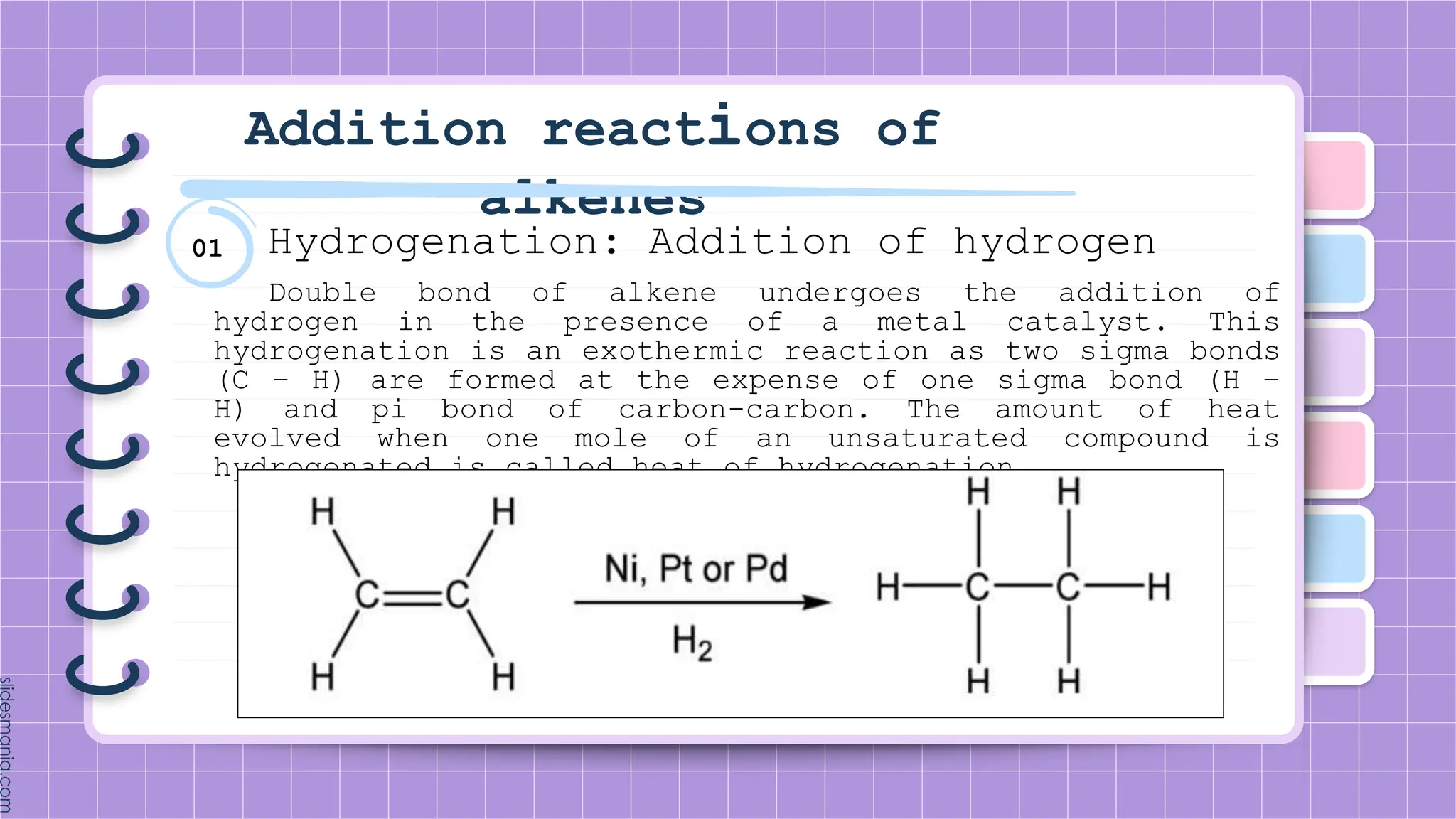
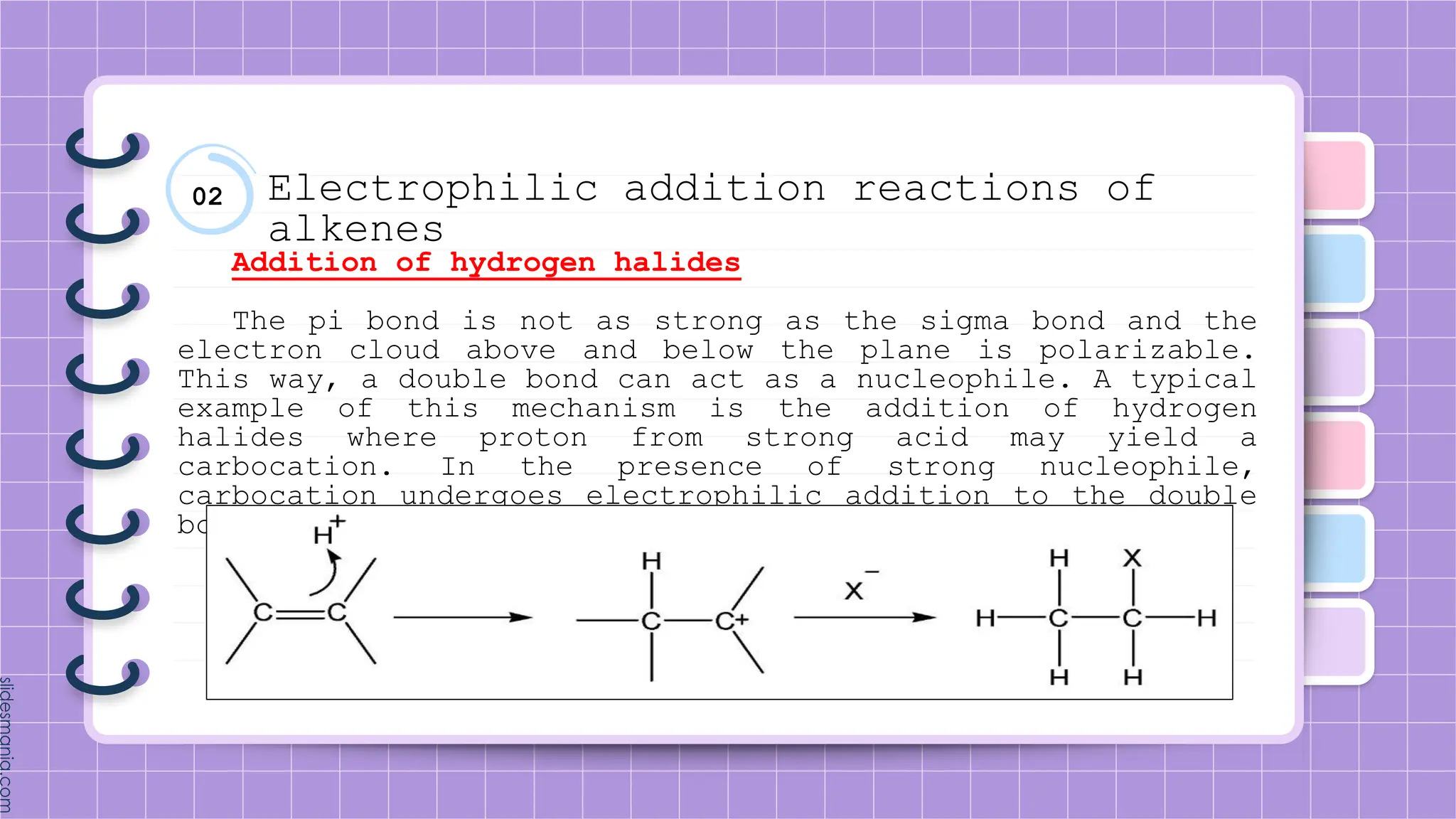
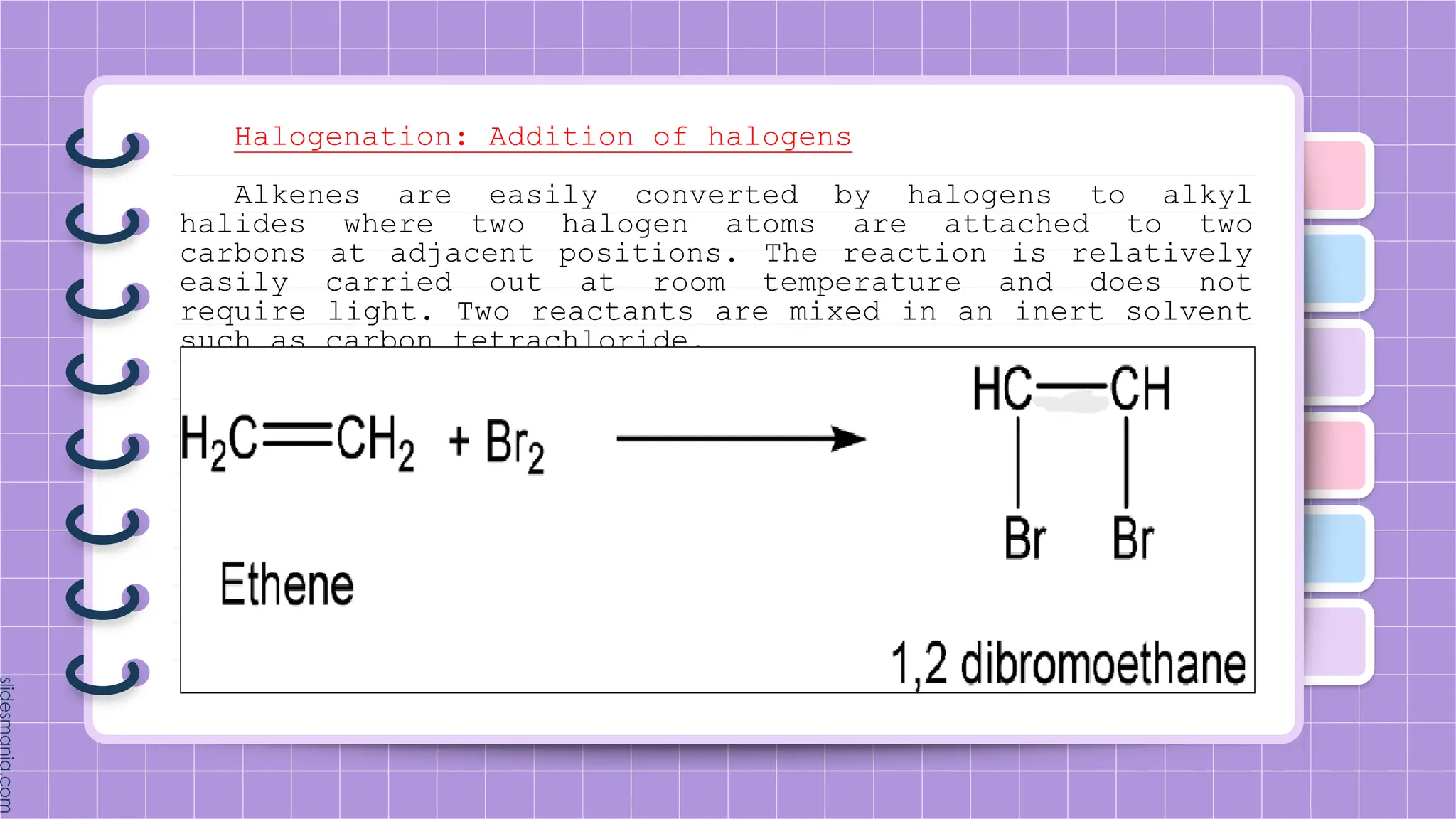
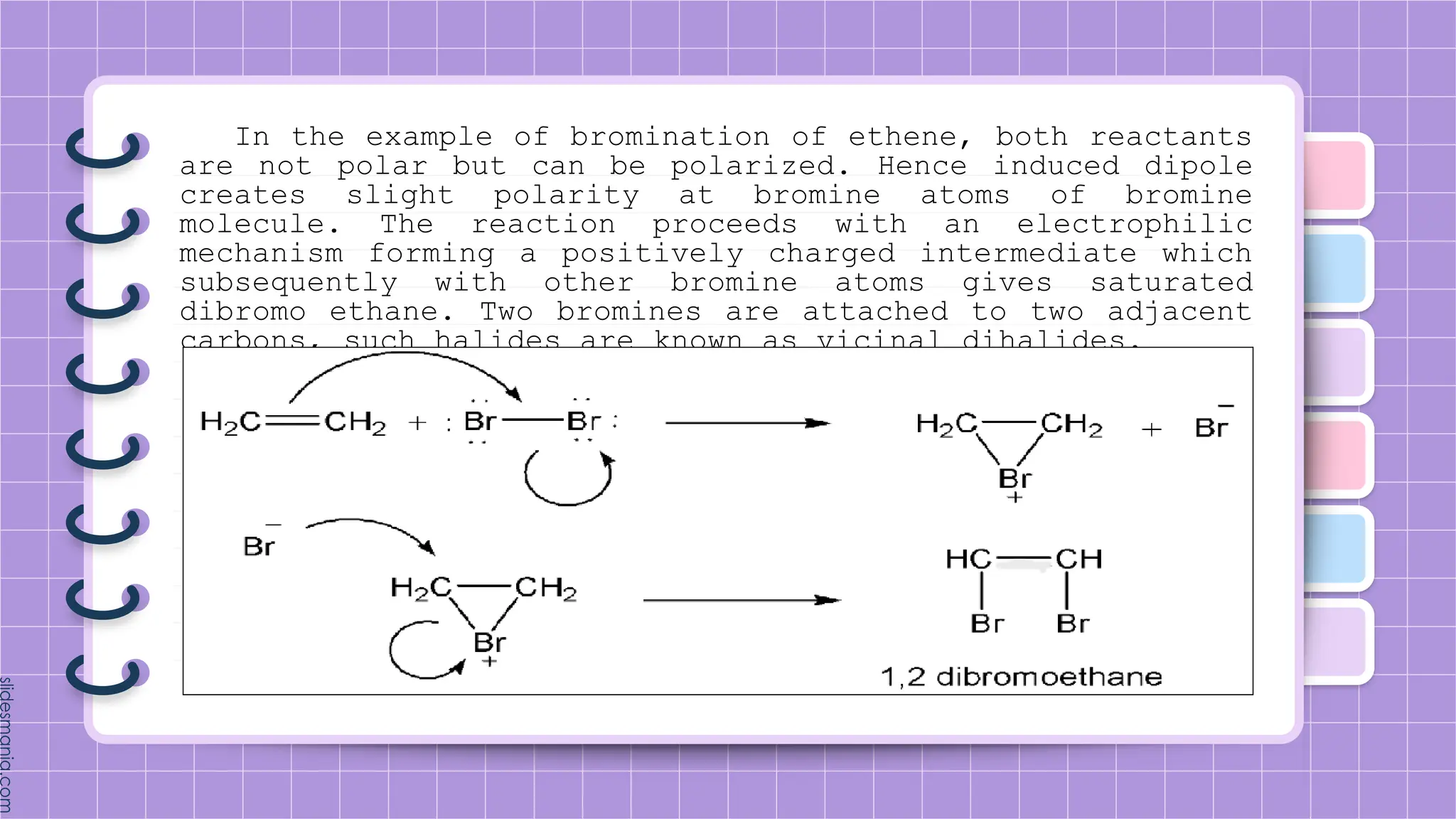
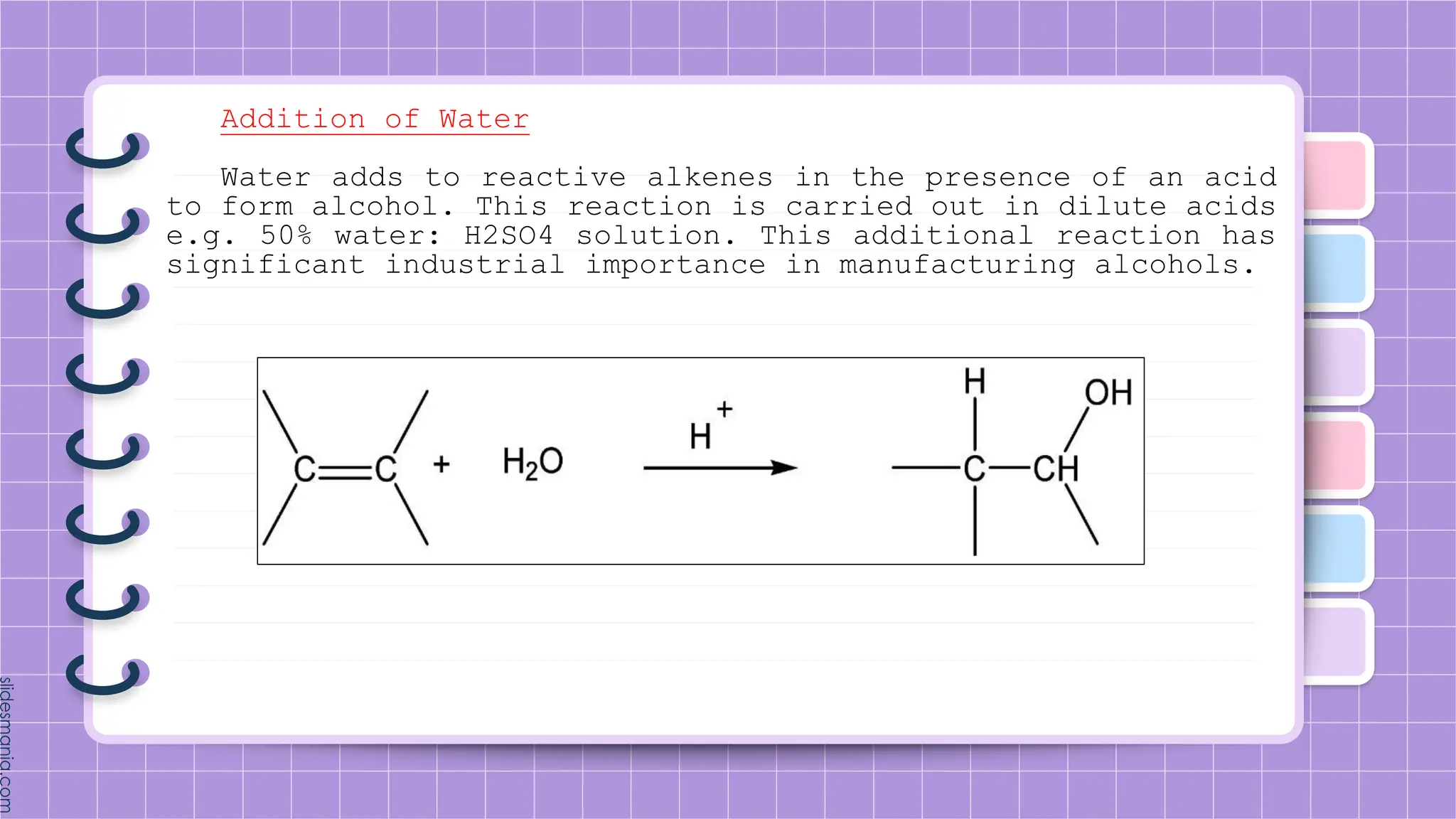
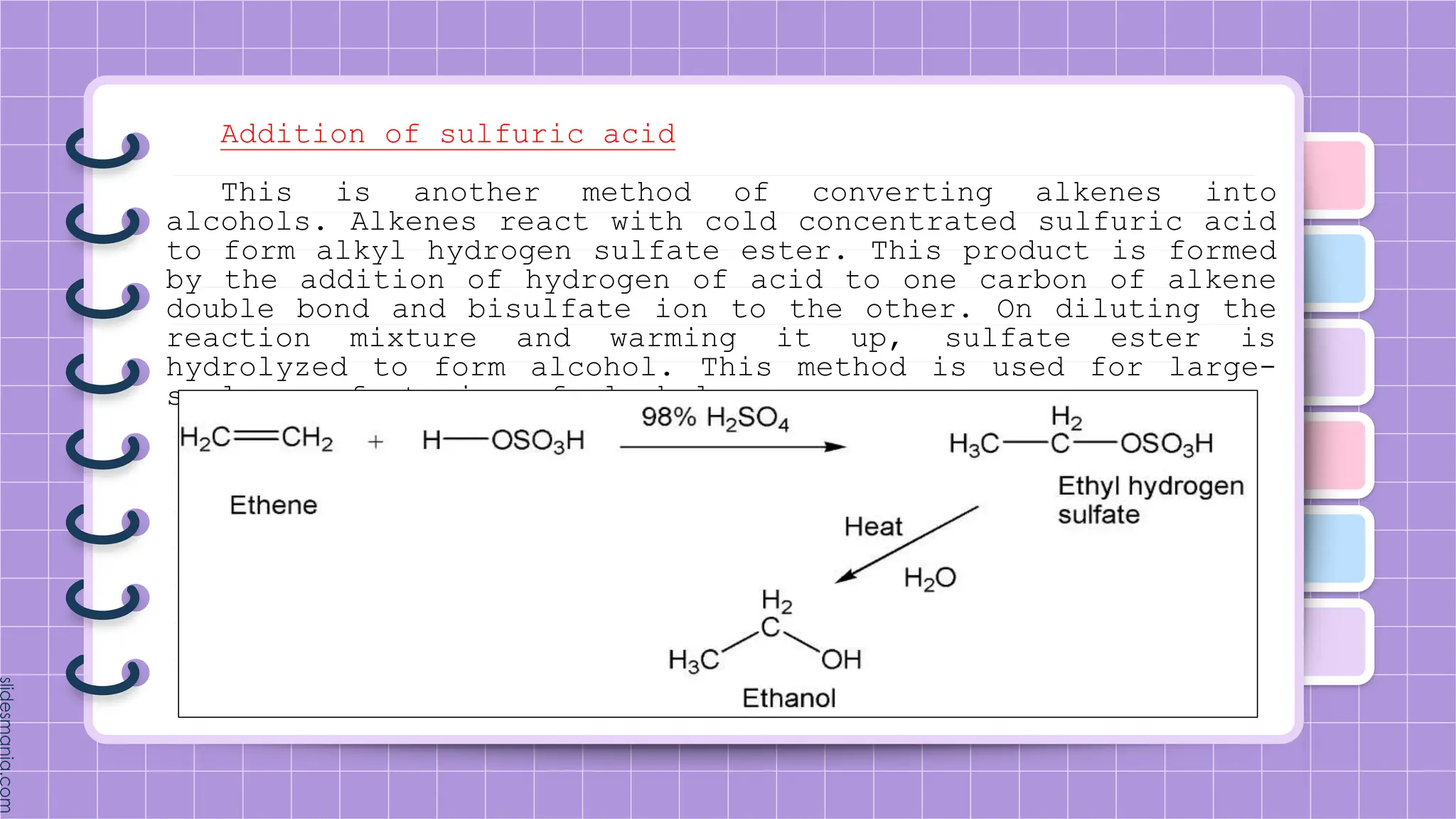
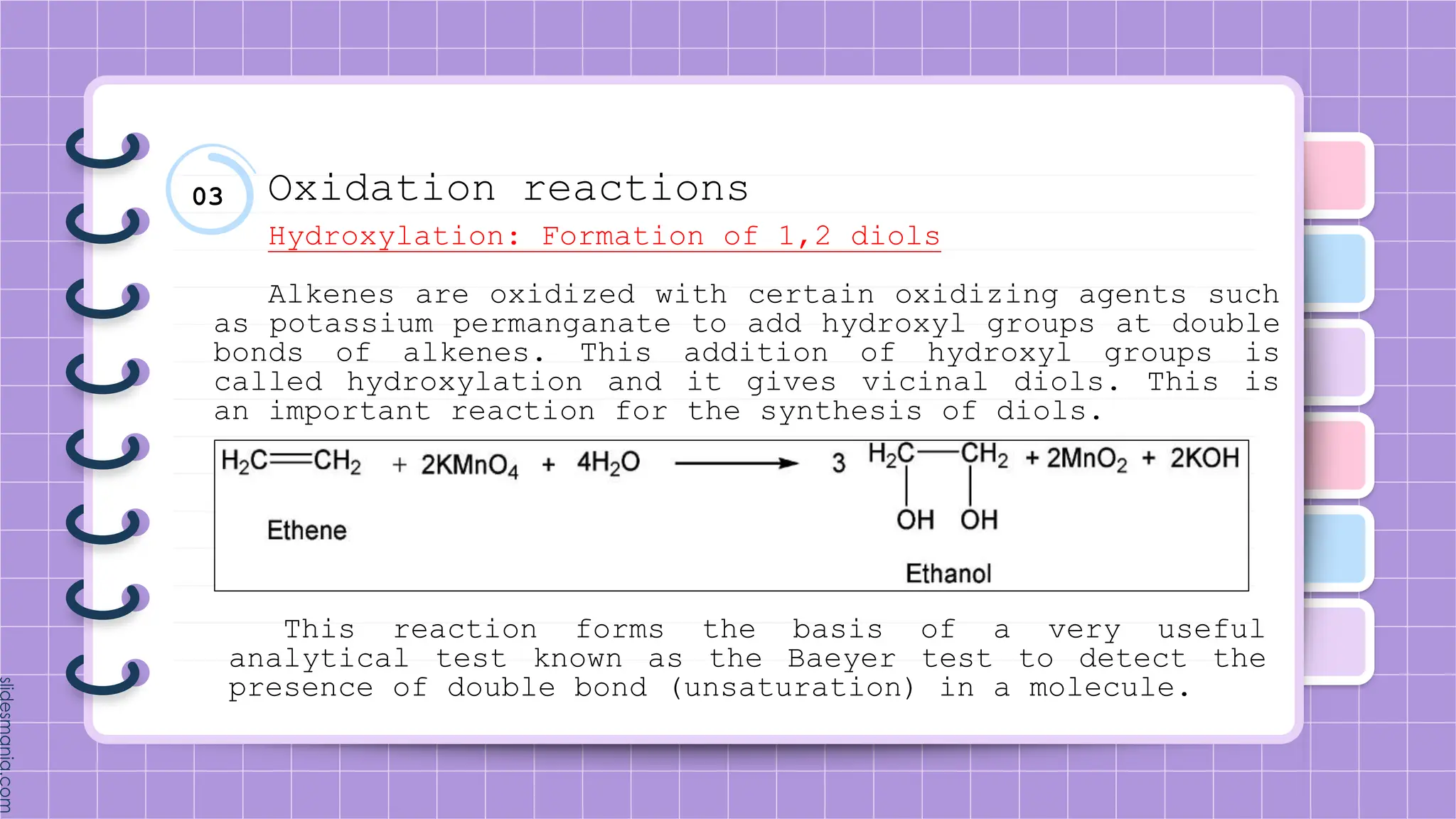
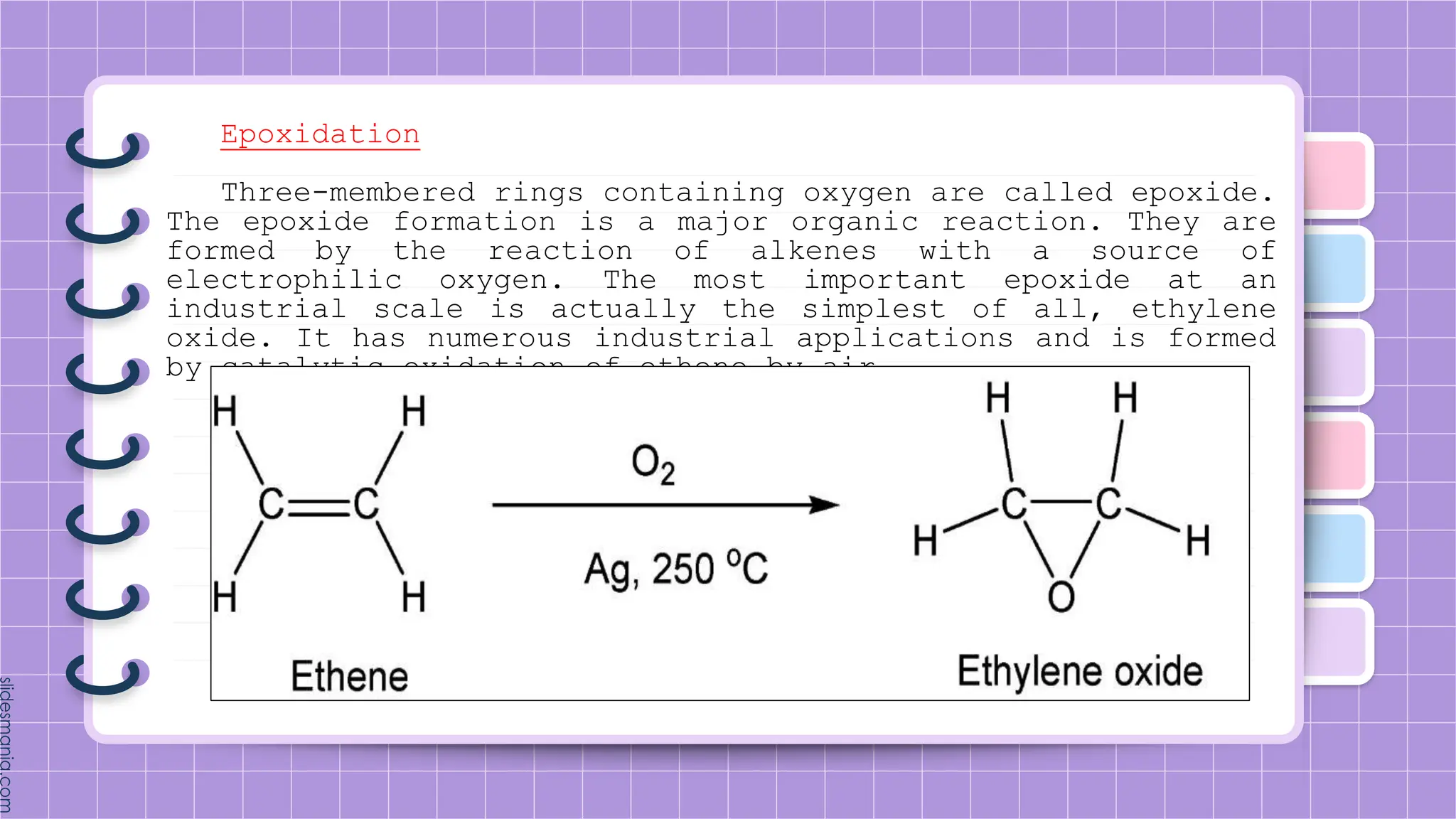
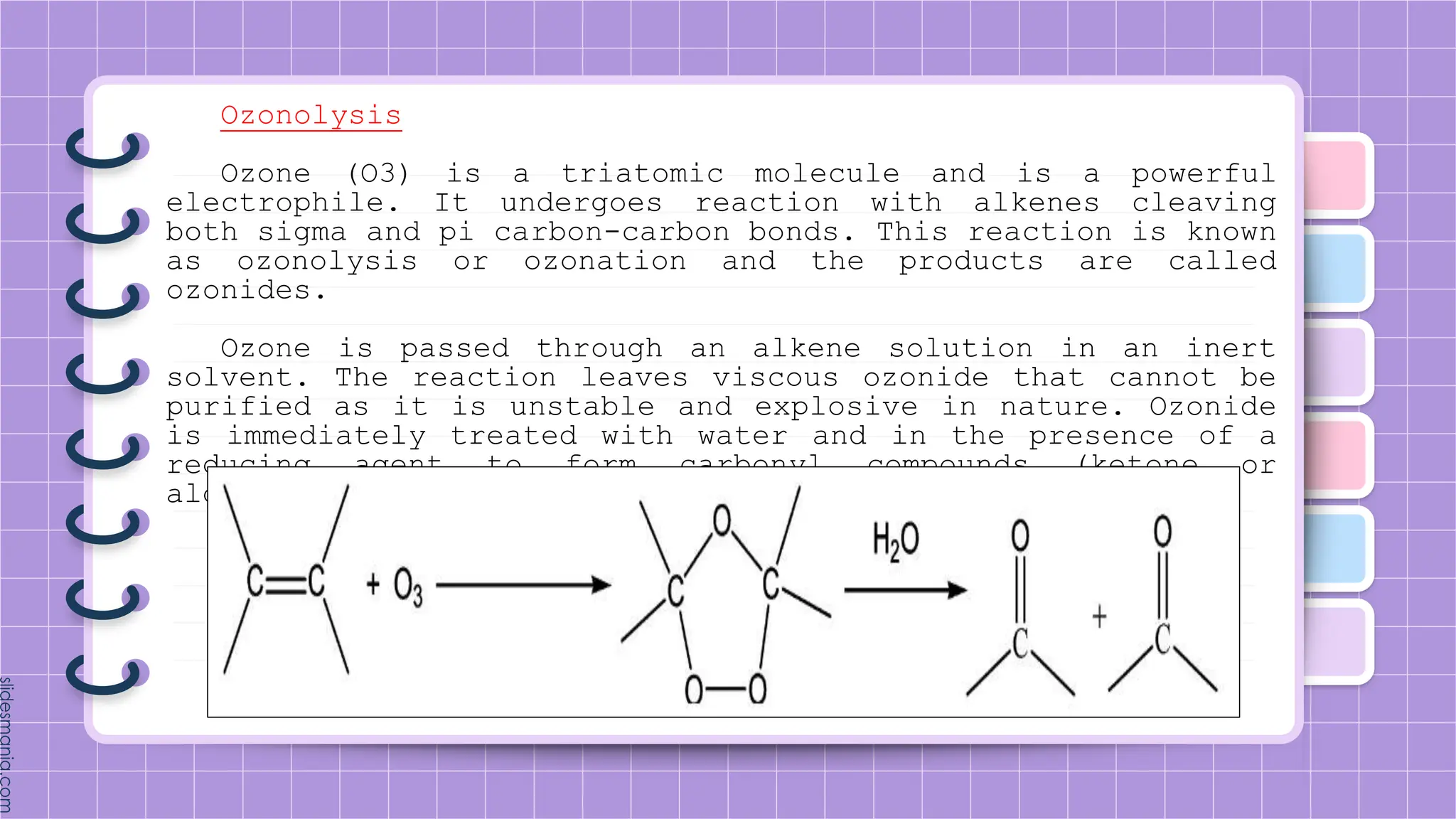
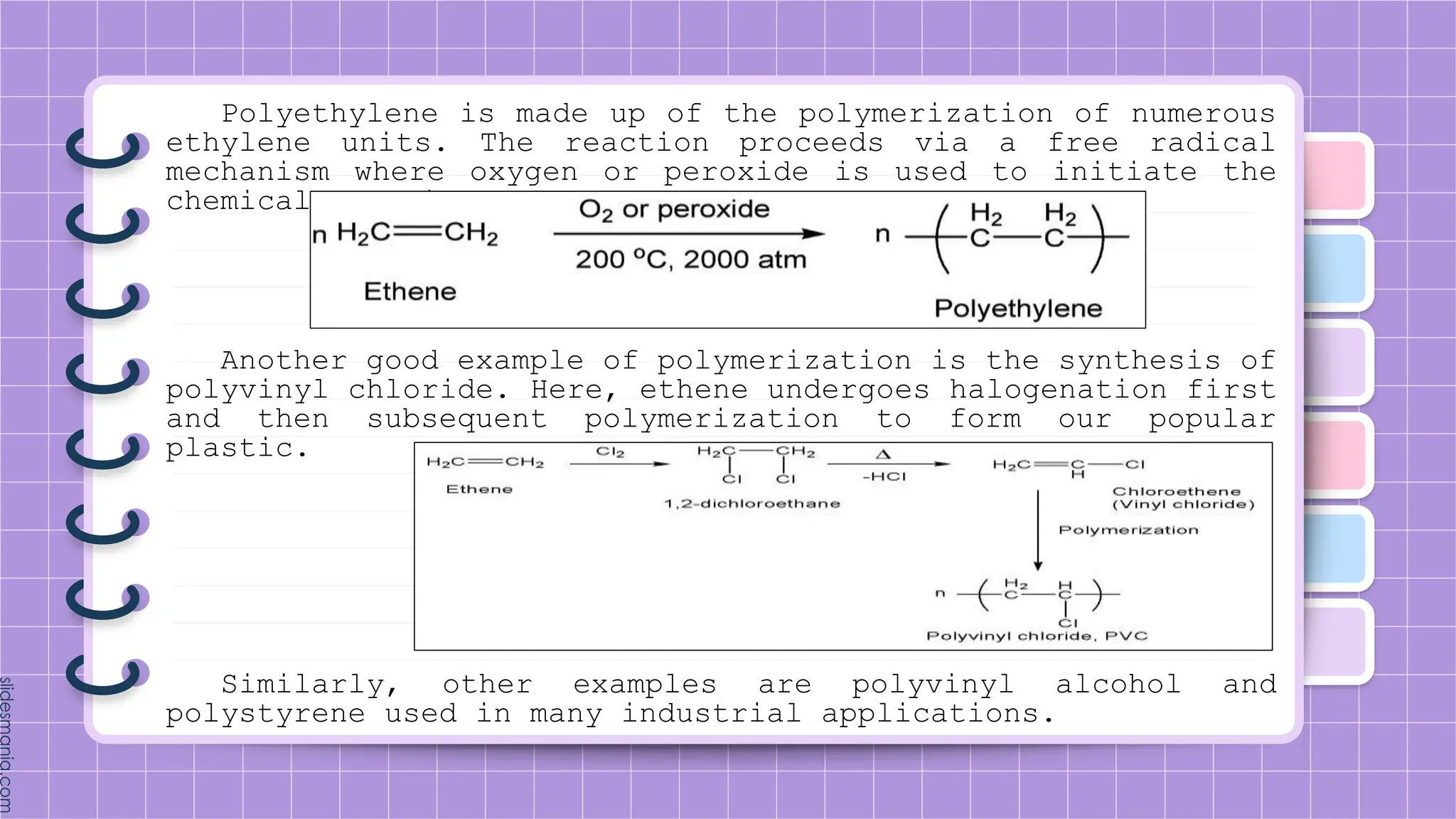
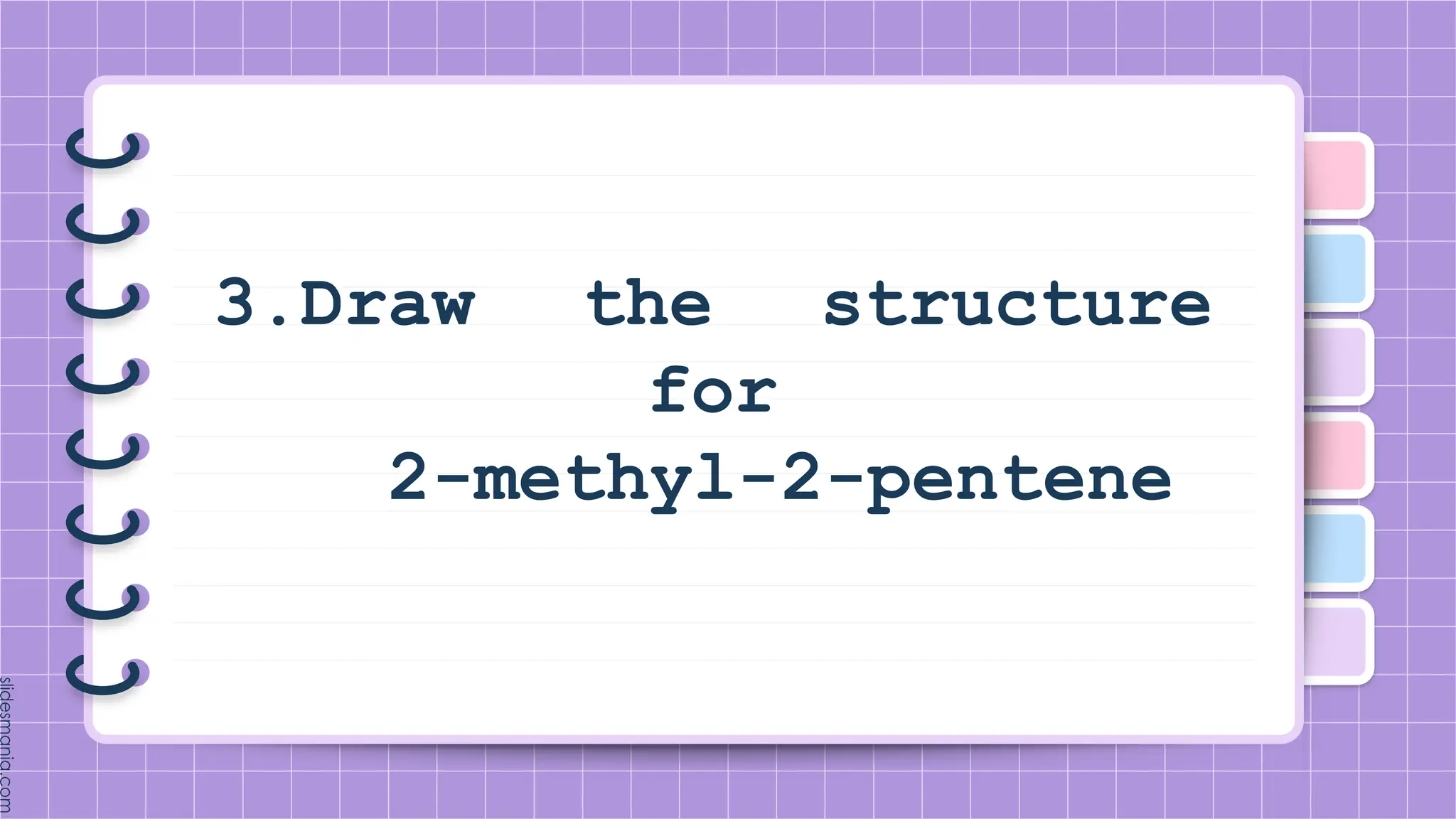
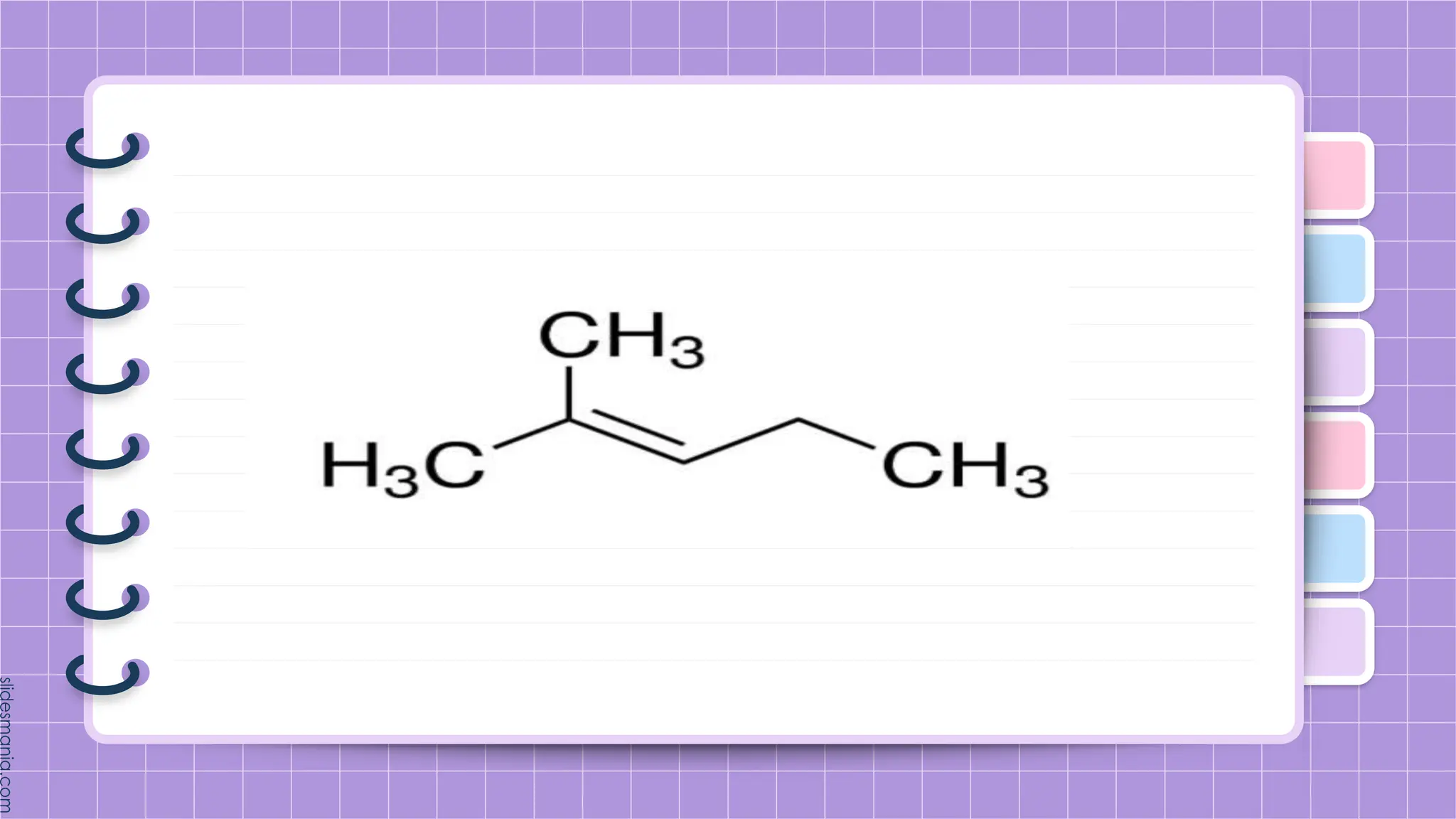
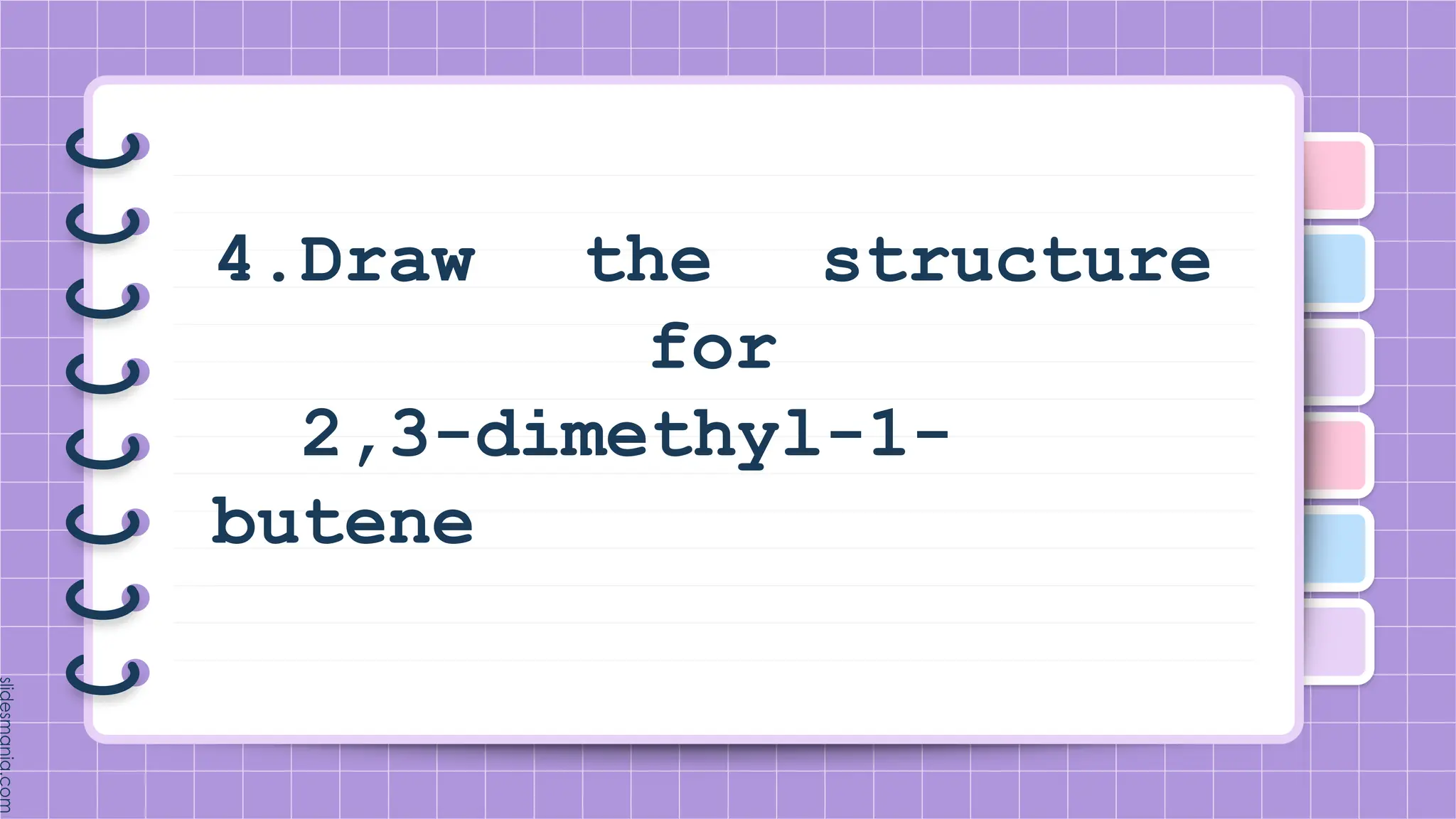
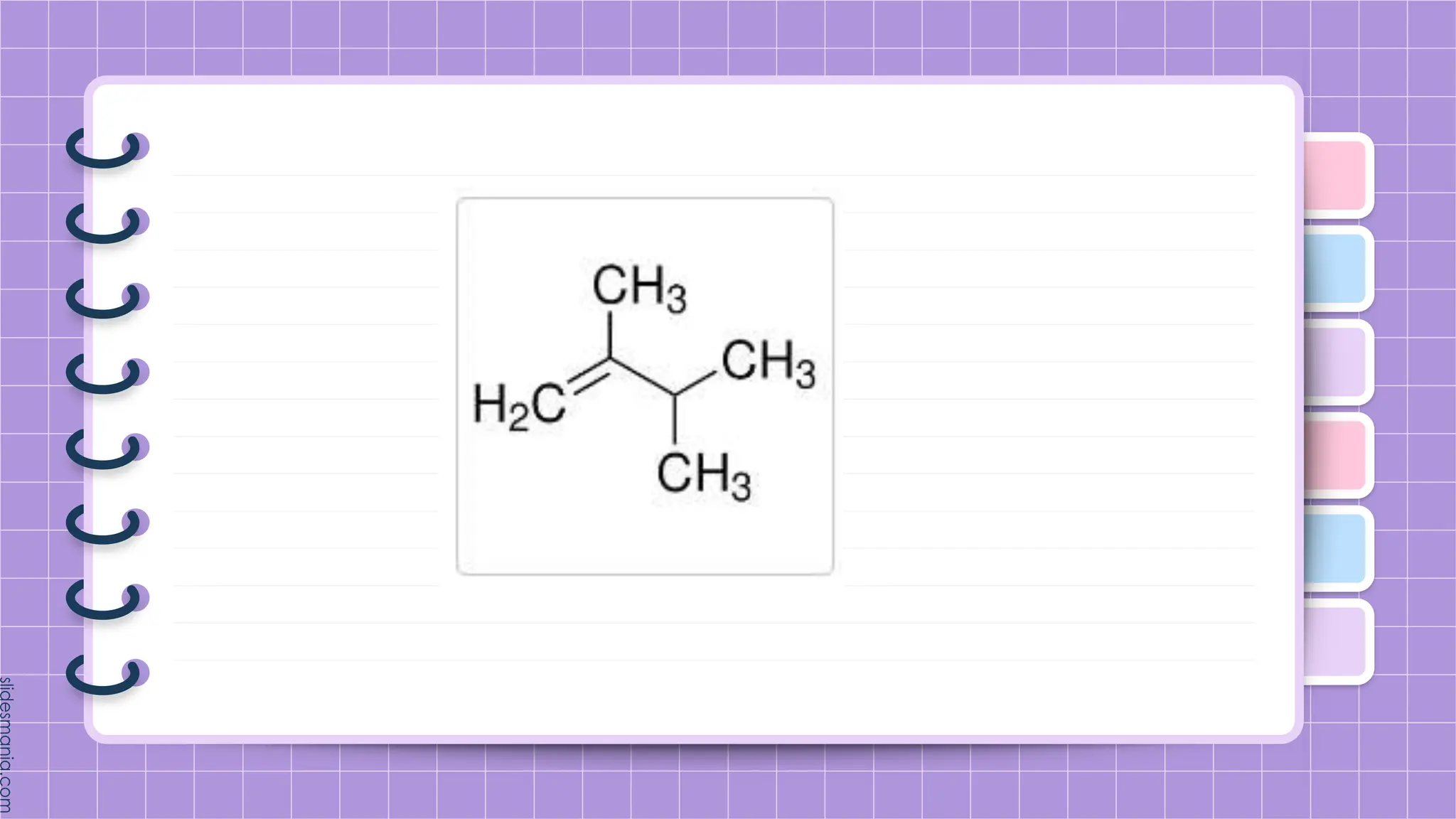
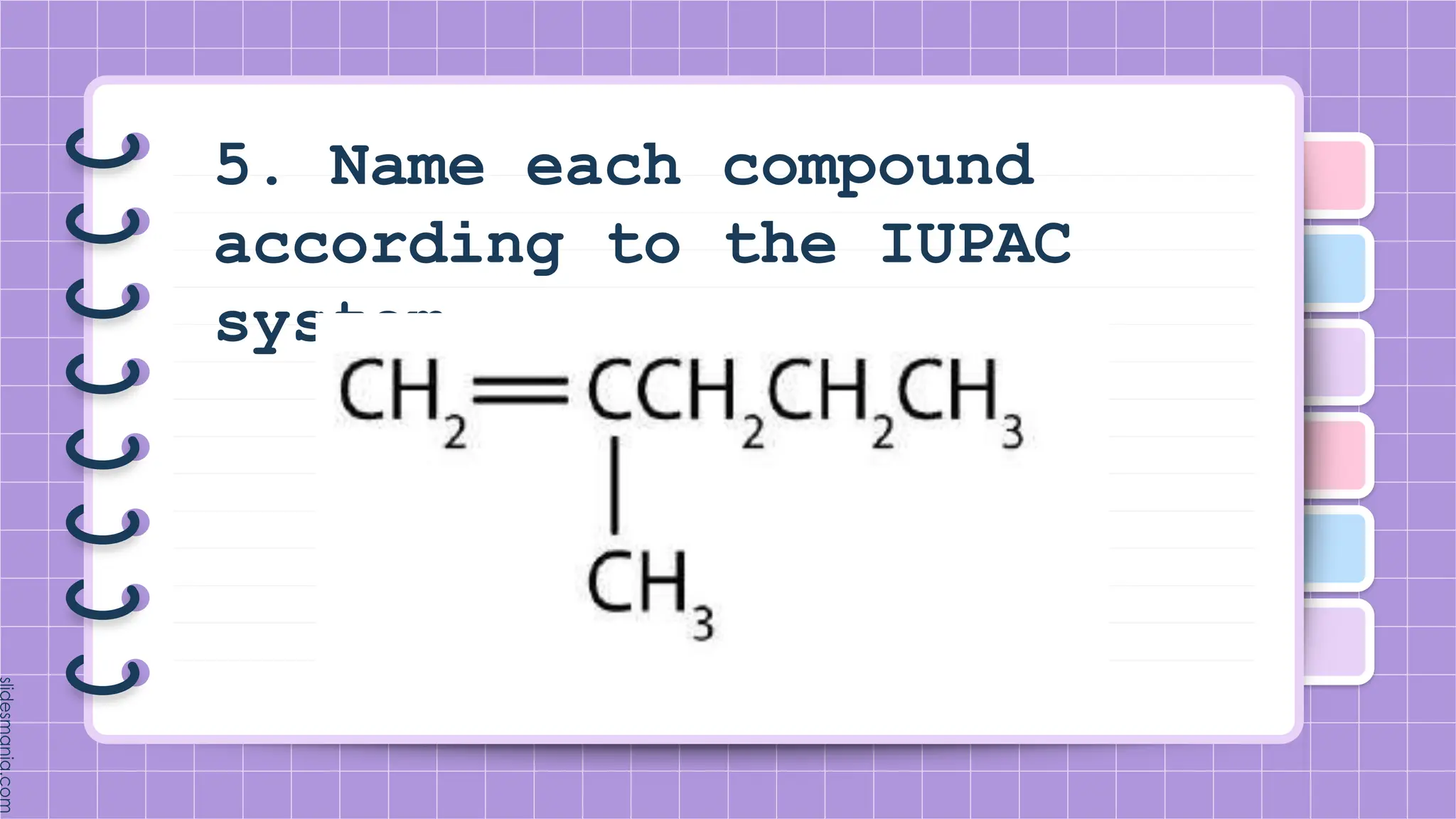
The document provides a comprehensive overview of alkenes, a class of unsaturated hydrocarbons characterized by at least one carbon-carbon double bond. It discusses their properties, structure, nomenclature, chemical reactions, and various applications, highlighting their significance in organic chemistry and everyday life. Key topics include physical and chemical properties, methods of naming, and the industrial importance of alkenes in materials and chemical synthesis.