Embed presentation

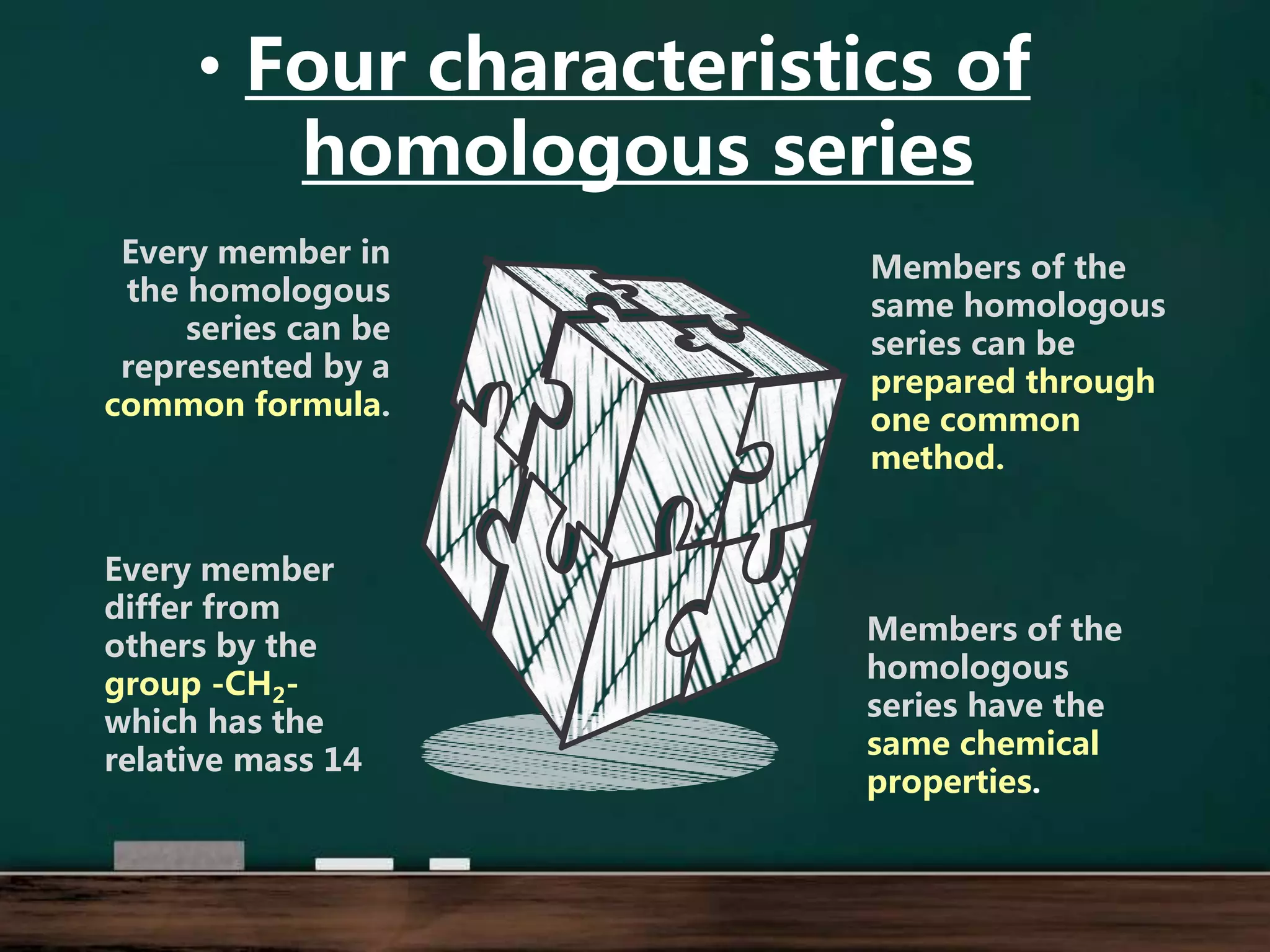





A homologous series is a series of compounds with similar chemical properties where each member differs from the next by a CH2 group. The key characteristics of a homologous series are: 1) Each member can be represented by a common chemical formula that differs by CH2. 2) Members are prepared by a common method. 3) Members have the same chemical properties. 4) Each member differs from the next by one CH2 group which has a mass of 14.






