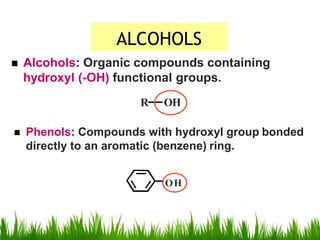
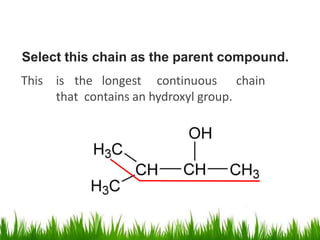
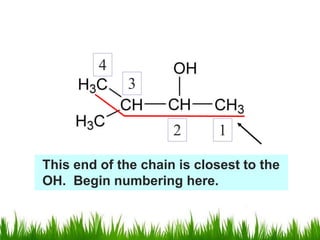
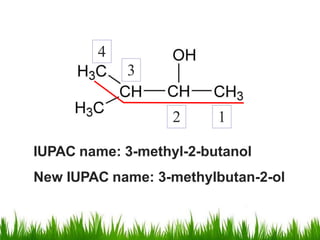
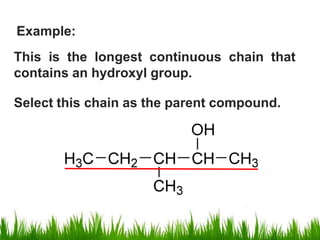
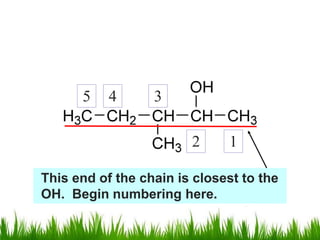
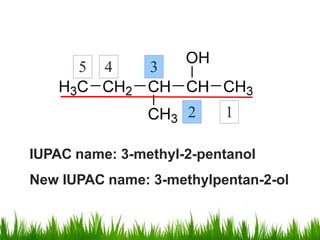
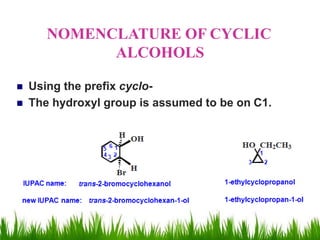
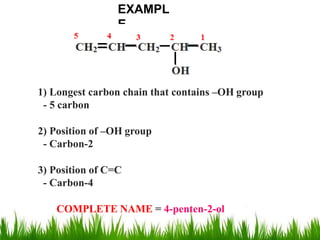
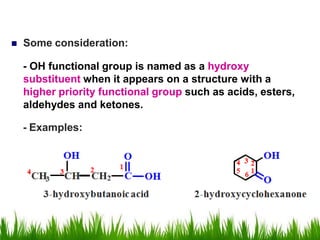
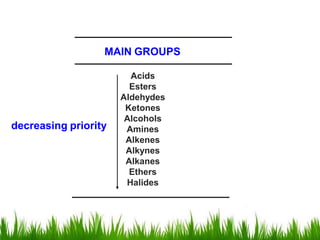
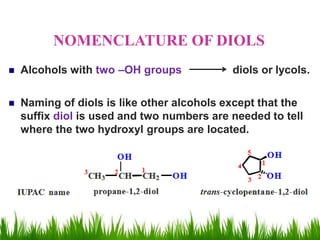
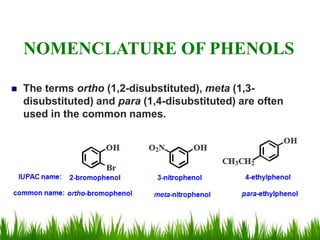
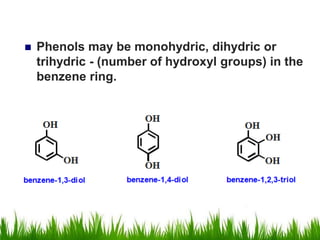
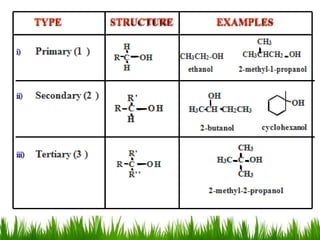
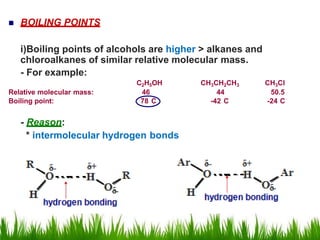
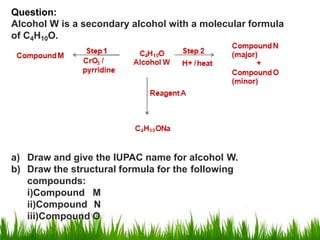
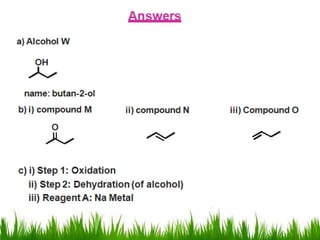
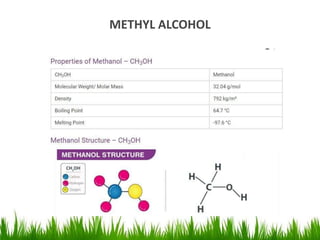
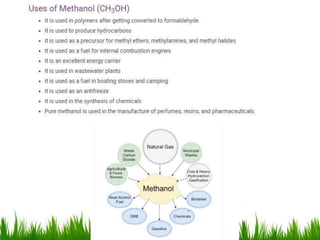
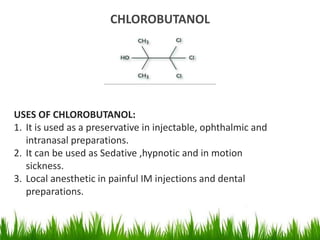
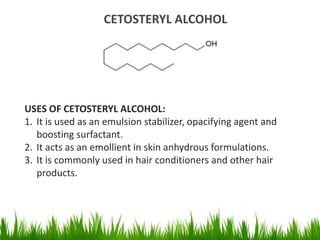
1) The document discusses the nomenclature, properties, preparation, and reactions of alcohols. It provides IUPAC rules for naming alcohols and describes substitutive and eliminative reactions.
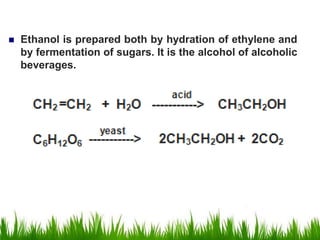
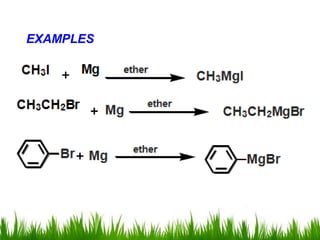
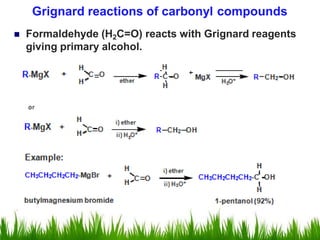
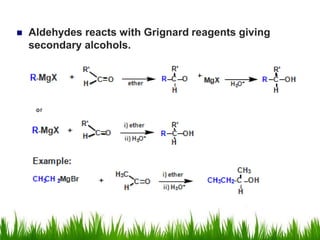
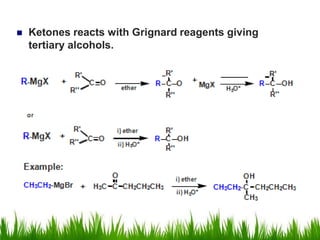
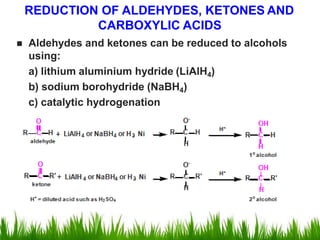
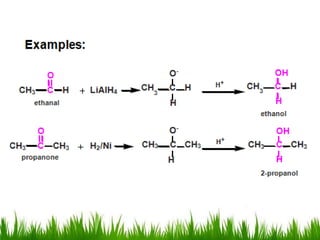
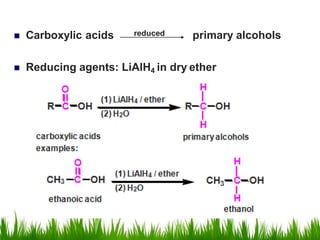
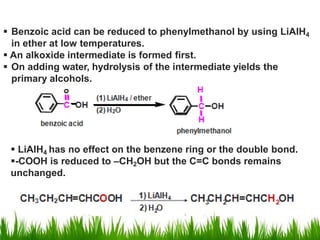
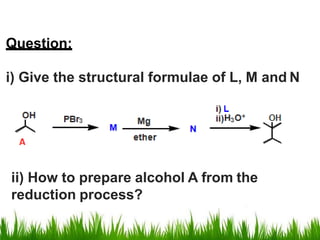
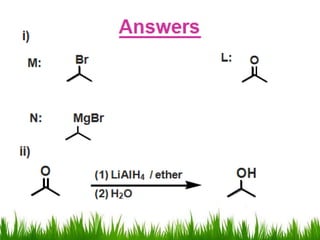
2) Alcohols are prepared through Grignard reactions with carbonyl compounds, hydrolysis of alkyl halides, and reduction of carbonyls with lithium aluminum hydride or sodium borohydride.
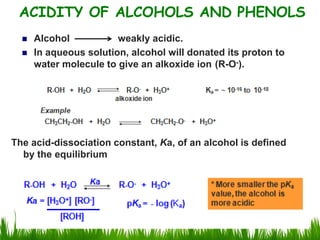
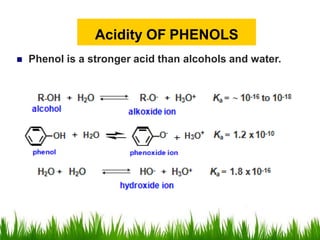
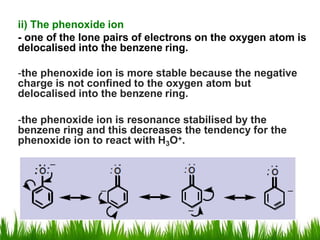
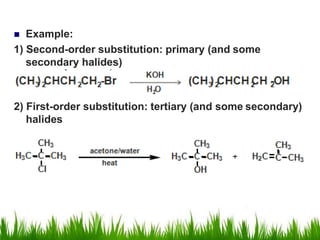
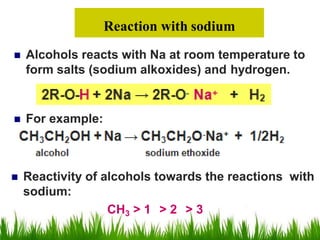
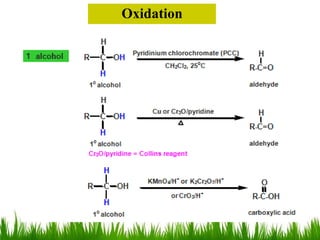
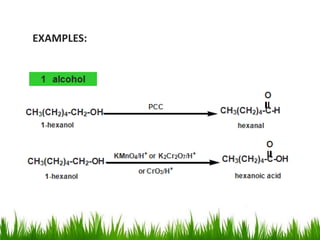
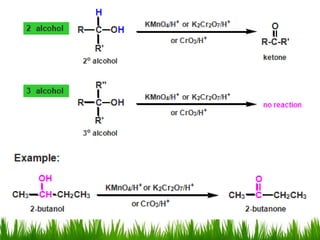
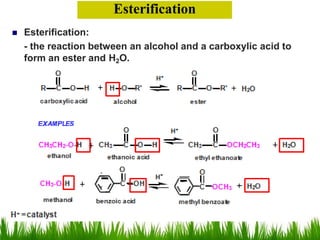
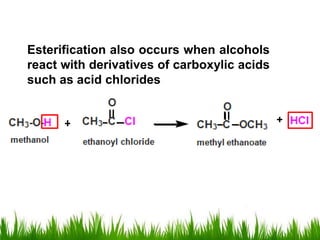
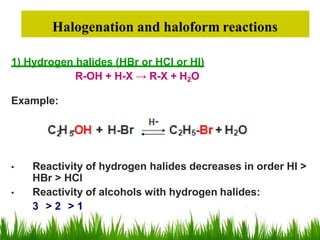
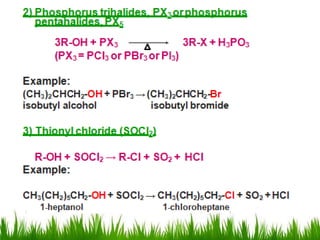
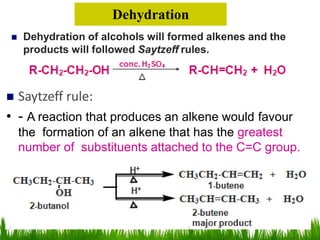
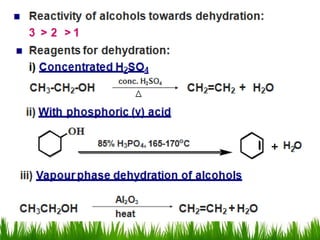
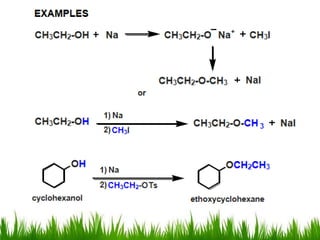
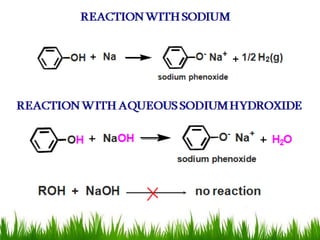
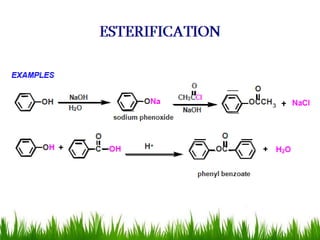
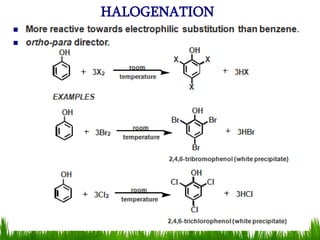
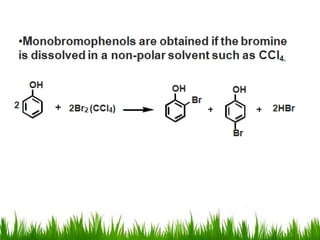
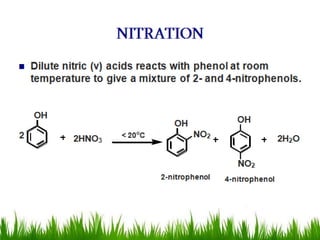
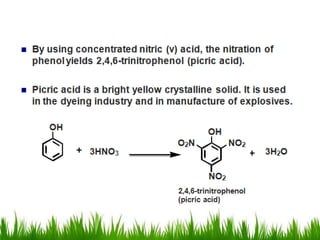
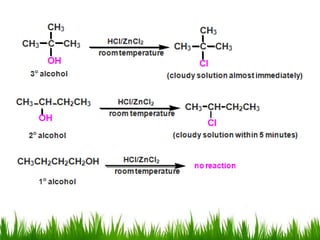
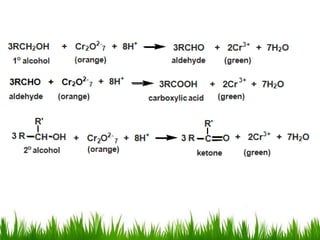
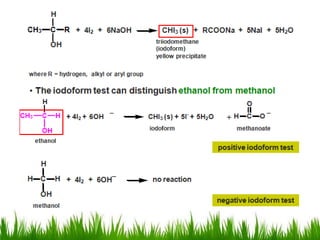
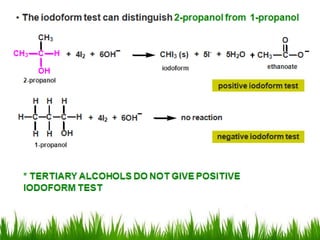
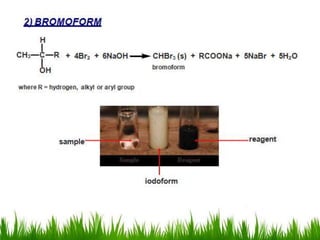
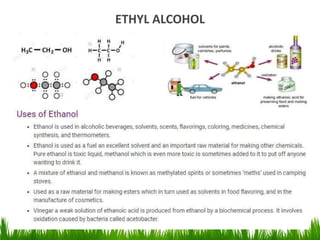
3) Alcohols undergo oxidation, esterification, halogenation, dehydration, and ether formation reactions. Primary alcohols react faster than secondary or tertiary alcohols in substitution and elimination reactions.