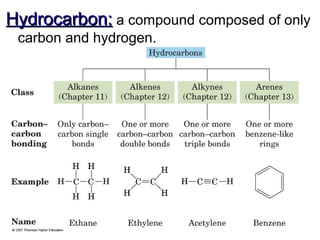
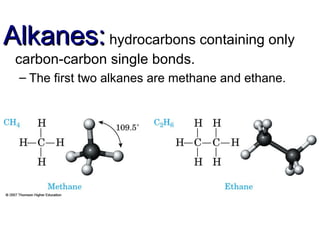
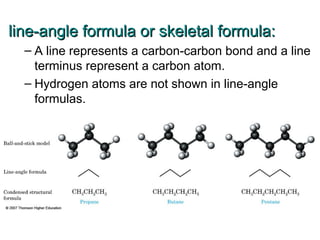
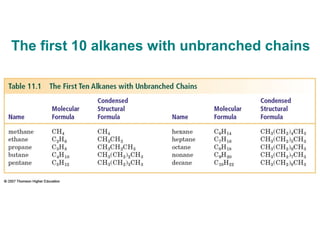
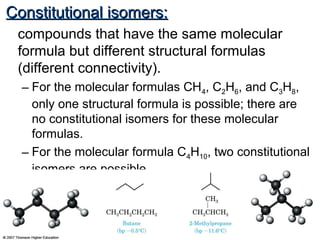
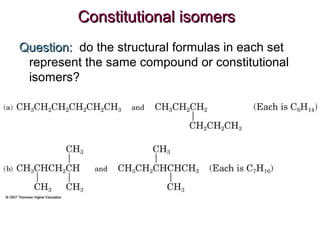
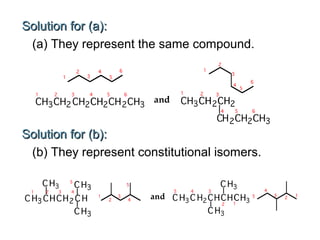
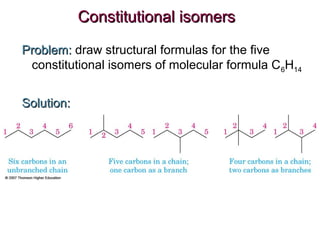
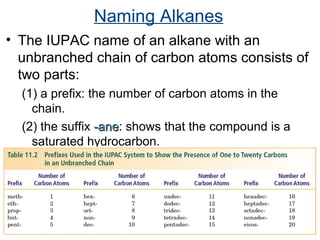
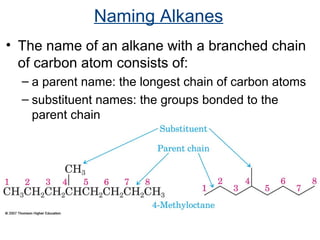
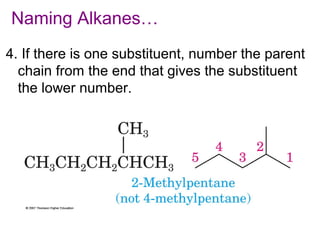
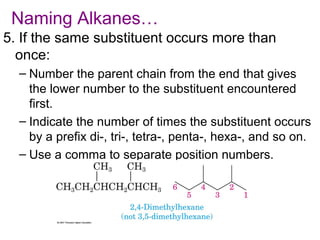
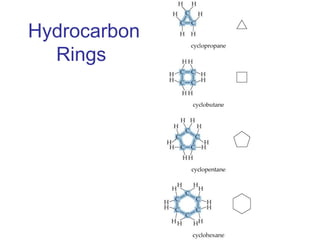
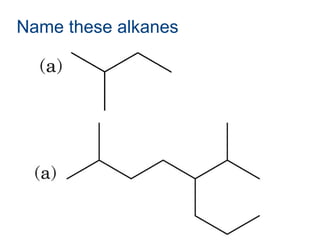
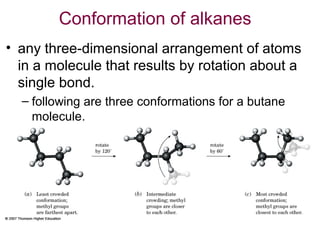
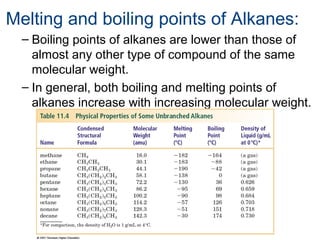
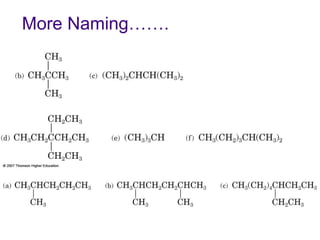
This document provides an overview of alkanes, including their structure, naming conventions, properties, and sources. It defines hydrocarbons and alkanes. Alkanes contain only single carbon-carbon bonds. Constitutional isomers are discussed. Naming conventions for alkanes include prefixes for carbon numbers and suffixes like -ane for straight chains or naming substituents on branches. Cycloalkanes are named similarly with the prefix cyclo-. Physical properties like boiling points increasing with molecular weight are covered. Alkanes are nonpolar and insoluble in water. Natural sources of hydrocarbons include natural gas and petroleum.