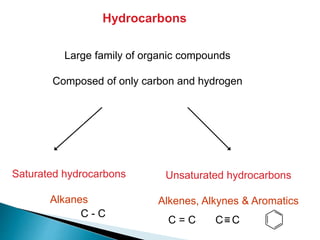
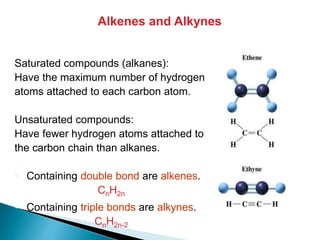
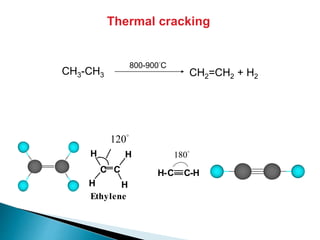
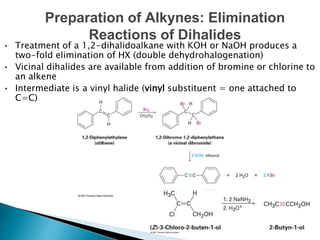
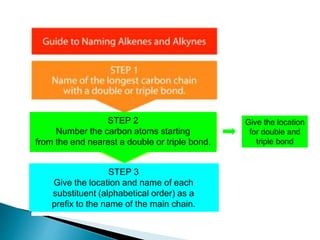
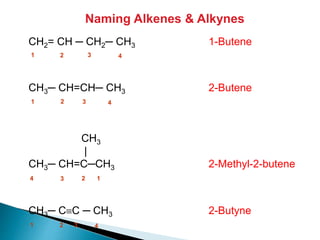
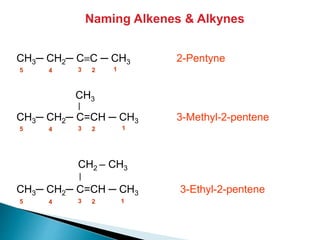
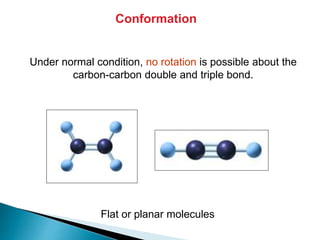
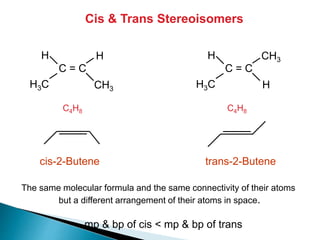
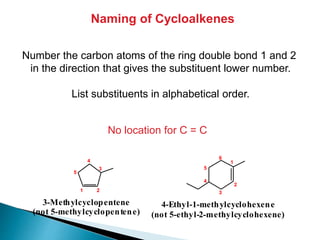
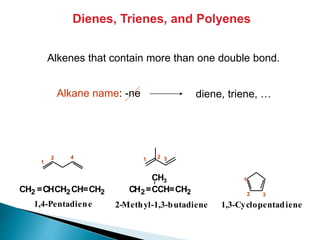
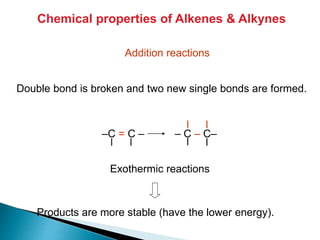
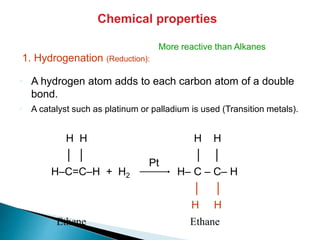
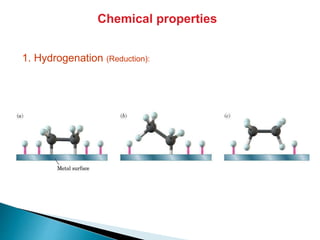
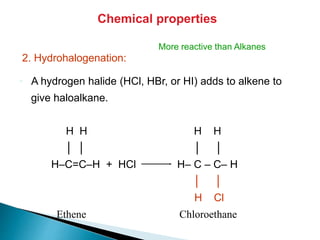
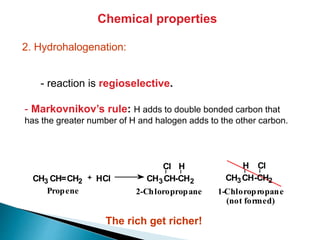
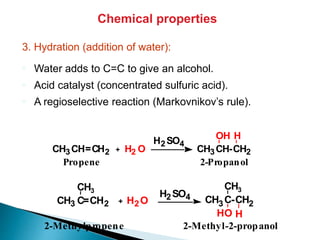
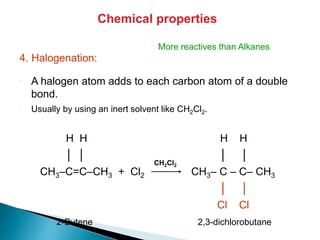
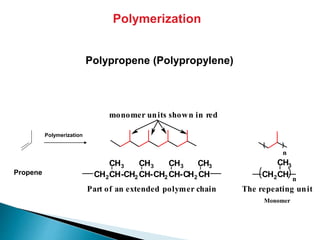
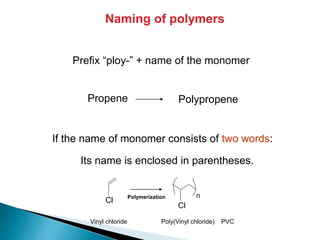
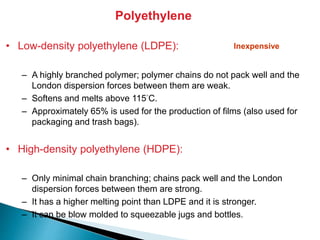
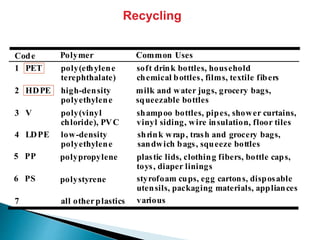
The document provides an overview of hydrocarbons, detailing the differences between saturated (alkanes) and unsaturated (alkenes, alkynes) compounds, their chemical properties, and reactions such as hydrogenation and hydrohalogenation. It also explains the nomenclature rules for naming alkenes and alkynes, the classification of cycloalkenes, and their reactivity including polymerization processes. Additionally, it highlights the application of various polymers in daily life and their associated properties.