Embed presentation
Downloaded 168 times
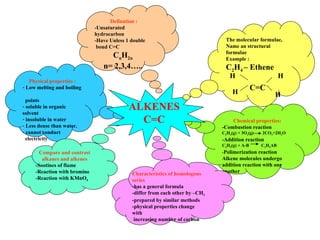

Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They have lower melting and boiling points than alkanes due to weaker intermolecular forces and are soluble in organic solvents but not water. Alkenes undergo combustion reactions, addition reactions, and polymerization reactions. They react with bromine water and potassium permanganate solution, showing characteristics of alkenes.
