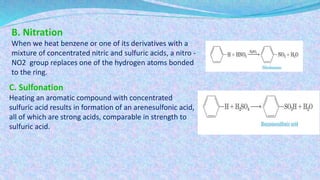
This document discusses aromatic compounds and benzene chemistry. It begins by introducing aromatic hydrocarbons and noting they have different properties than aliphatic hydrocarbons. Benzene, the simplest aromatic hydrocarbon, is described as having posed problems for early chemists to determine its structure. Kekulé proposed benzene has alternating single and double bonds, but this did not explain its chemical behavior. The resonance structure of benzene is able to account for its reactivity. The document continues discussing nomenclature of aromatic compounds with different numbers of substituents on the benzene ring. Characteristic reactions of benzene like halogenation and nitration are also covered. Phenols are introduced as aromatic compounds containing an -OH group