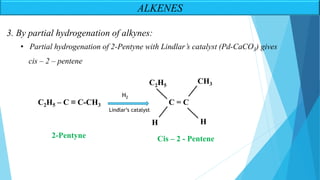
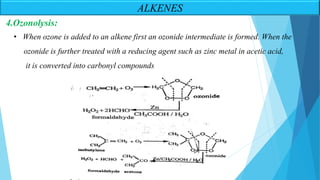
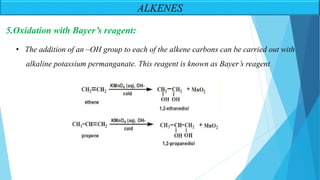
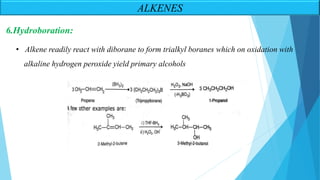
The document outlines unsaturated hydrocarbons, specifically focusing on alkenes, dienes, and alkynes, detailing their definitions, general formulas, preparation methods, and chemical properties. It explains the mechanisms of alkene formation through dehydration and dehydrohalogenation, as well as addition reactions and oxidation processes. Additionally, it classifies dienes based on double bond arrangements and discusses alkynes, including their preparation and reactivity with various chemical agents.









![Step 4: Since Secondary free radical is more stable than primary free radical, it will be formed
predominately and will react with another molecule of HBr to yield the anti –
markownikoff’s product as major product
• The resulted bromine radical will further react with another molecule of propylene to
continue the chain
CH3 - CH .–CH2 –Br + HBr CH3CH2CH2Br + Br .
1-Bromopropane
[Major product]
ALKENES](https://image.slidesharecdn.com/alkenesandalkynes-210605095523/85/Alkenes-and-alkynes-12-320.jpg)



![Compounds containing two double bonds are called dienes
Example: H2C=CH-CH=CH2 [1,3 - butadiene]
General formula : CnH2n-2
Types of dienes:
Depending on the relative location of the double bonds, dienes can be divided into
three types
1. Cumulated dienes
2. Conjugated dienes
3. Isolated dienes
Definition:
DIENES](https://image.slidesharecdn.com/alkenesandalkynes-210605095523/85/Alkenes-and-alkynes-19-320.jpg)
![• Dienes in which two double bonds are shared by a common carbon atom are called
cumulated dienes
Example: H2C=C=CH2 [Propadiene]
1.Cumulated dienes:
• Dienes in which two double bonds are separated by one single bond are called
conjugated dienes
Example: H2C=CH-CH=CH2 [1,3 - butadiene]
2.Conjugated dienes:
DIENES](https://image.slidesharecdn.com/alkenesandalkynes-210605095523/85/Alkenes-and-alkynes-20-320.jpg)
![• Dienes in which two double bonds are separated by two or more single bonds are called
Isolated dienes
Example: H2C=CH-CH2-CH2-CH=CH2 [1,5 - Hexadiene]
3.Isolated dienes:
• By dehydrogenation of alkene (1-Butene) by passing the vapour over heated catalyst. Cr2O3
on alumina support at 800-900 K
Preparation of dienes from alkene:
H2C=CH-CH2-CH3 H2C=CH-CH=CH2
Cr2O3 /Alumina
800 – 900 K
1-Butene 1,3 -Butadiene
DIENES](https://image.slidesharecdn.com/alkenesandalkynes-210605095523/85/Alkenes-and-alkynes-21-320.jpg)