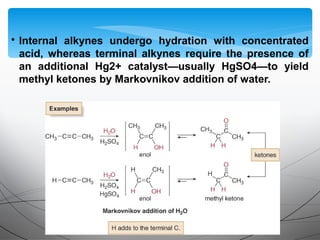
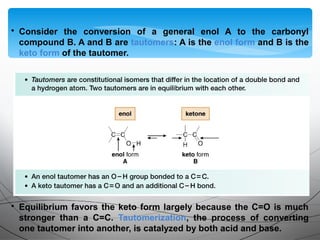
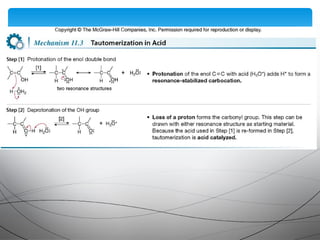
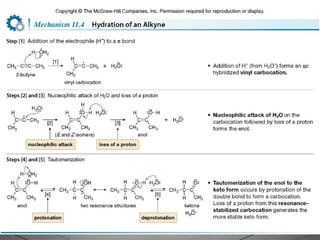
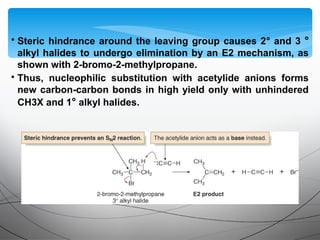
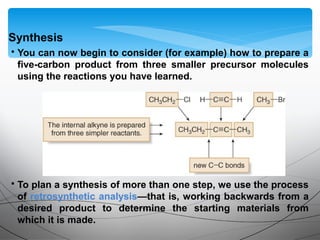
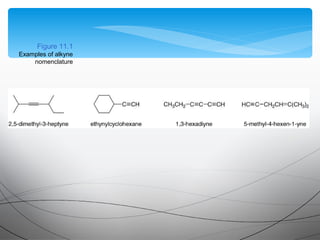
This document provides an overview of alkynes, including their structure, nomenclature, properties, reactions, and synthesis. Key points include:
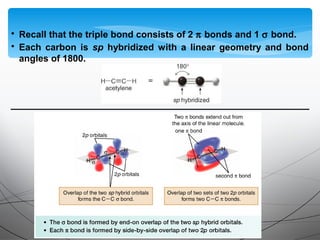
- Alkynes contain a triple bond consisting of two pi bonds and one sigma bond, giving them a linear structure.
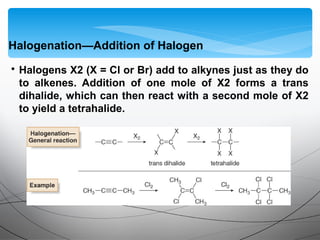
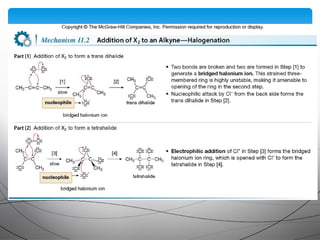
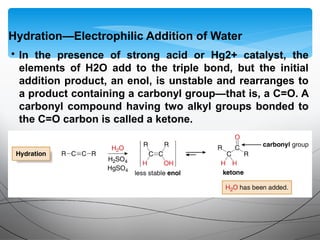
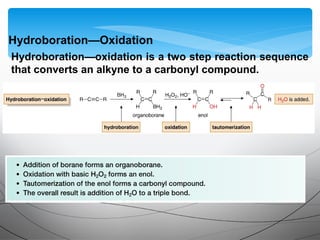
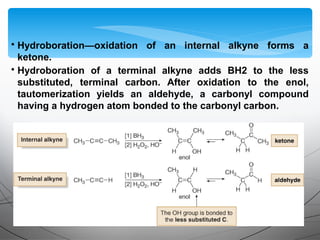
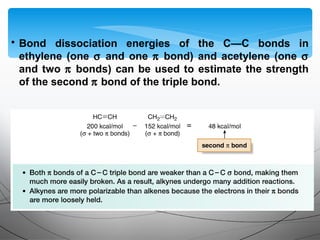
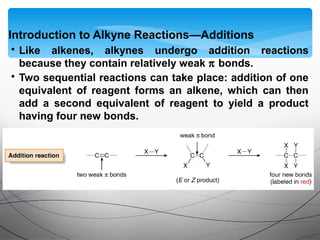
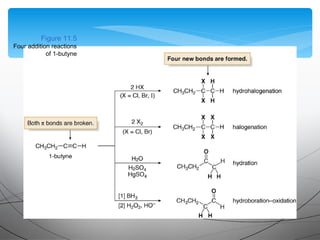
- They undergo addition reactions due to their relatively weak pi bonds. Common additions include hydrohalogenation, hydration, halogenation, and hydroboration-oxidation.
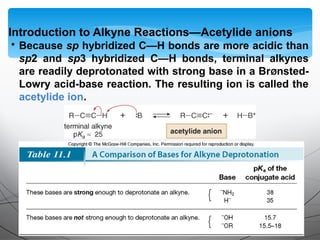
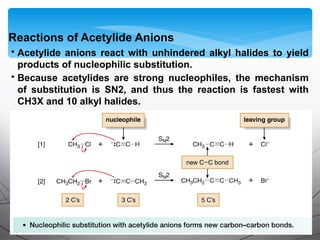
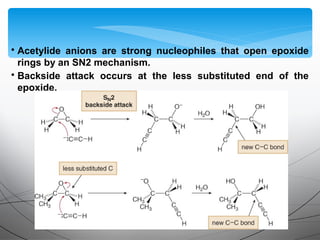
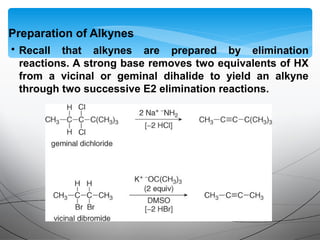
- Acetylide ions, formed by deprotonation of terminal alkynes, are strong nucleophiles that react through substitution and addition reactions.


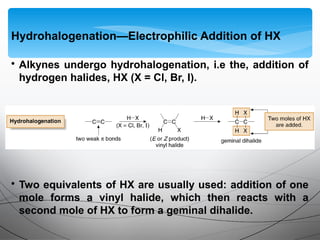

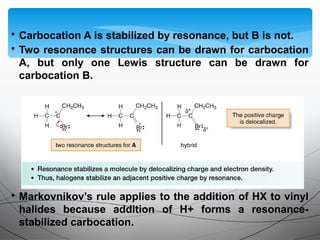
![• Electrophilic addition of HX to alkynes is slower than
electrophilic addition of HX to alkenes, even though alkynes are
more polarizable and have more loosely held π electrons than
alkenes.
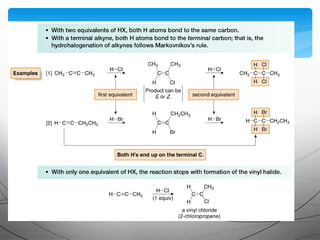
• Markovnikov addition in step [3] places the H on the terminal
carbon to form the more substituted carbocation A, rather than
the less substituted carbocation B.](https://image.slidesharecdn.com/aadilali10-190221062422/85/Alkynes-15-320.jpg)