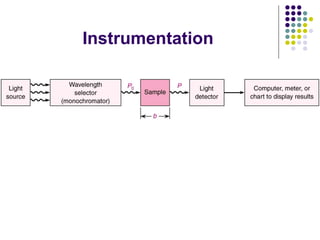
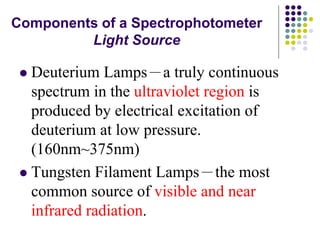
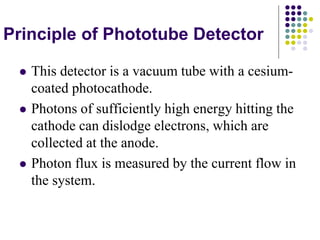
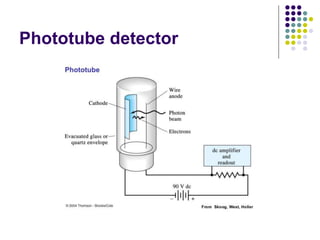
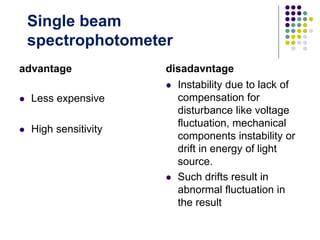
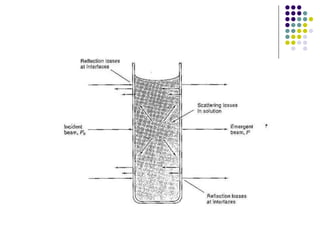
Beer's law and Lambert's law describe the relationship between light absorption and the properties of the material that the light passes through. Specifically, Beer's law states that absorption increases exponentially with increasing concentration or path length, while Lambert's law states it increases exponentially with path length alone. Together they form Beer-Lambert law. A spectrophotometer uses these principles to measure the absorption of light by a sample to identify unknown substances or determine concentrations. It has components like a light source, monochromator, and detector to measure transmittance or absorbance. Single beam spectrometers measure one path at a time, while double beam measures a sample and reference simultaneously for better accuracy.