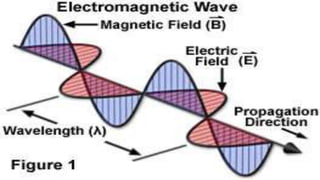
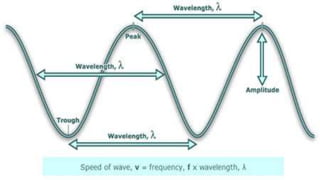
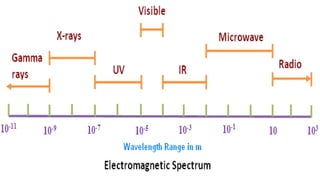
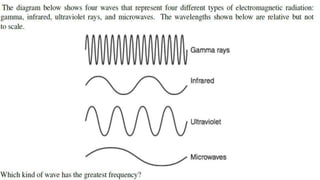
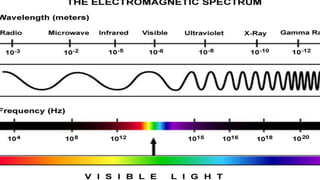
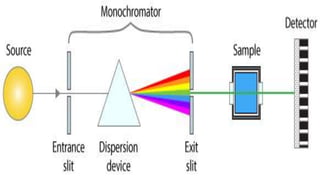
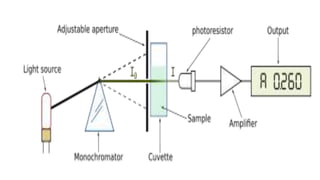
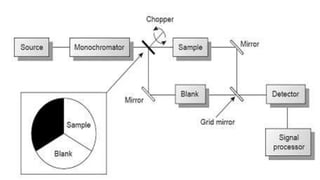
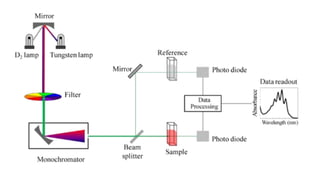
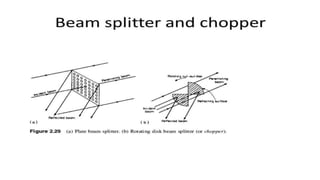
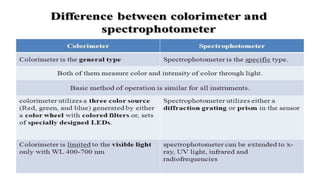
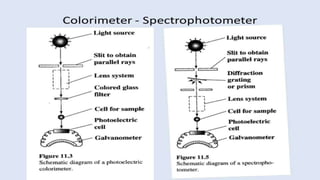
Spectroscopy is the study of the interaction between electromagnetic radiation and matter. A spectrometer is used to measure the presence of compounds in a molecule by analyzing the spectrum produced when matter interacts with different wavelengths of light. Absorption spectroscopy involves matter absorbing radiation and undergoing an electronic transition to a higher energy state. UV/visible spectroscopy uses this technique to study electronic transitions in atoms and molecules in the ultraviolet and visible light ranges.