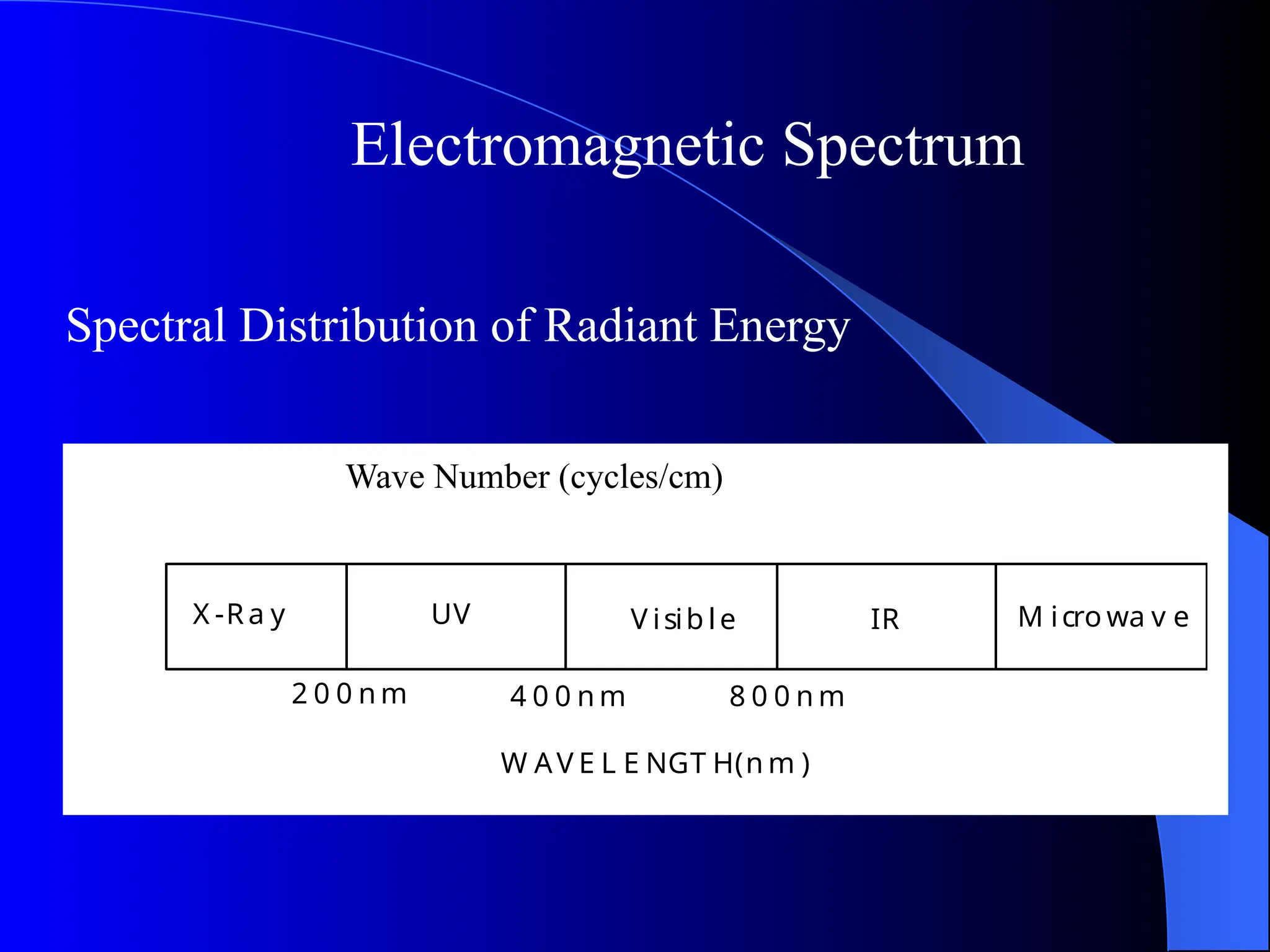
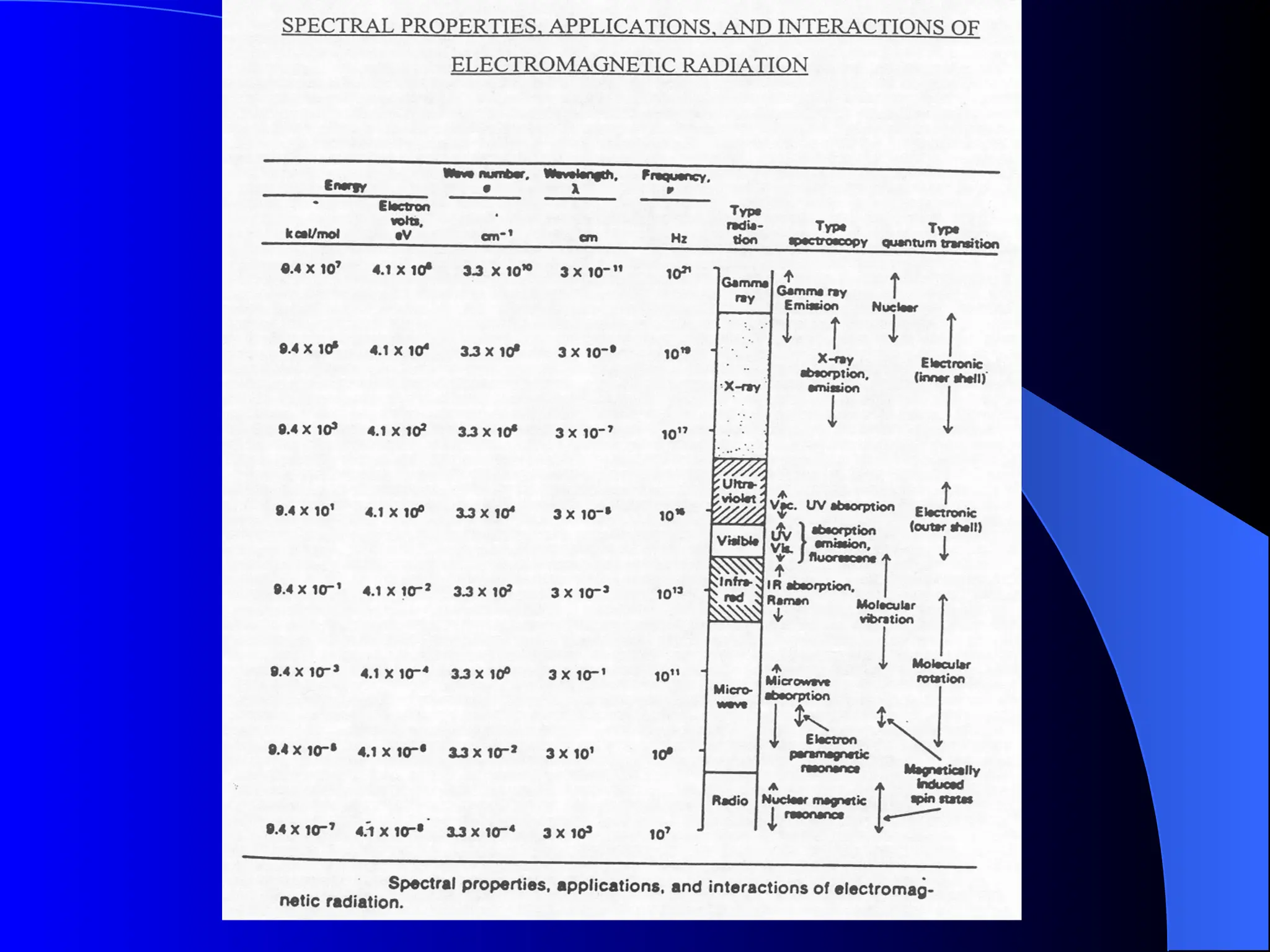
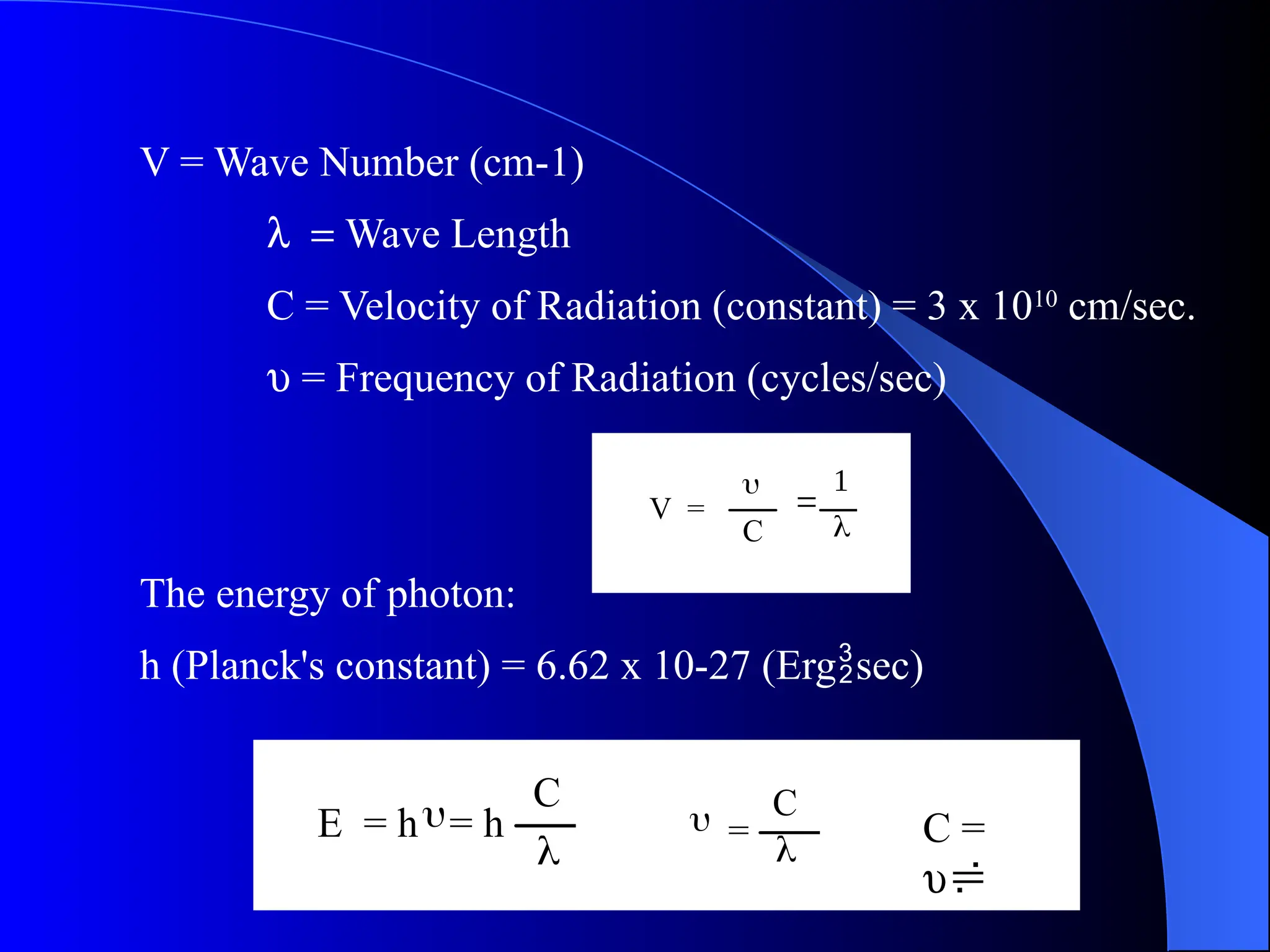
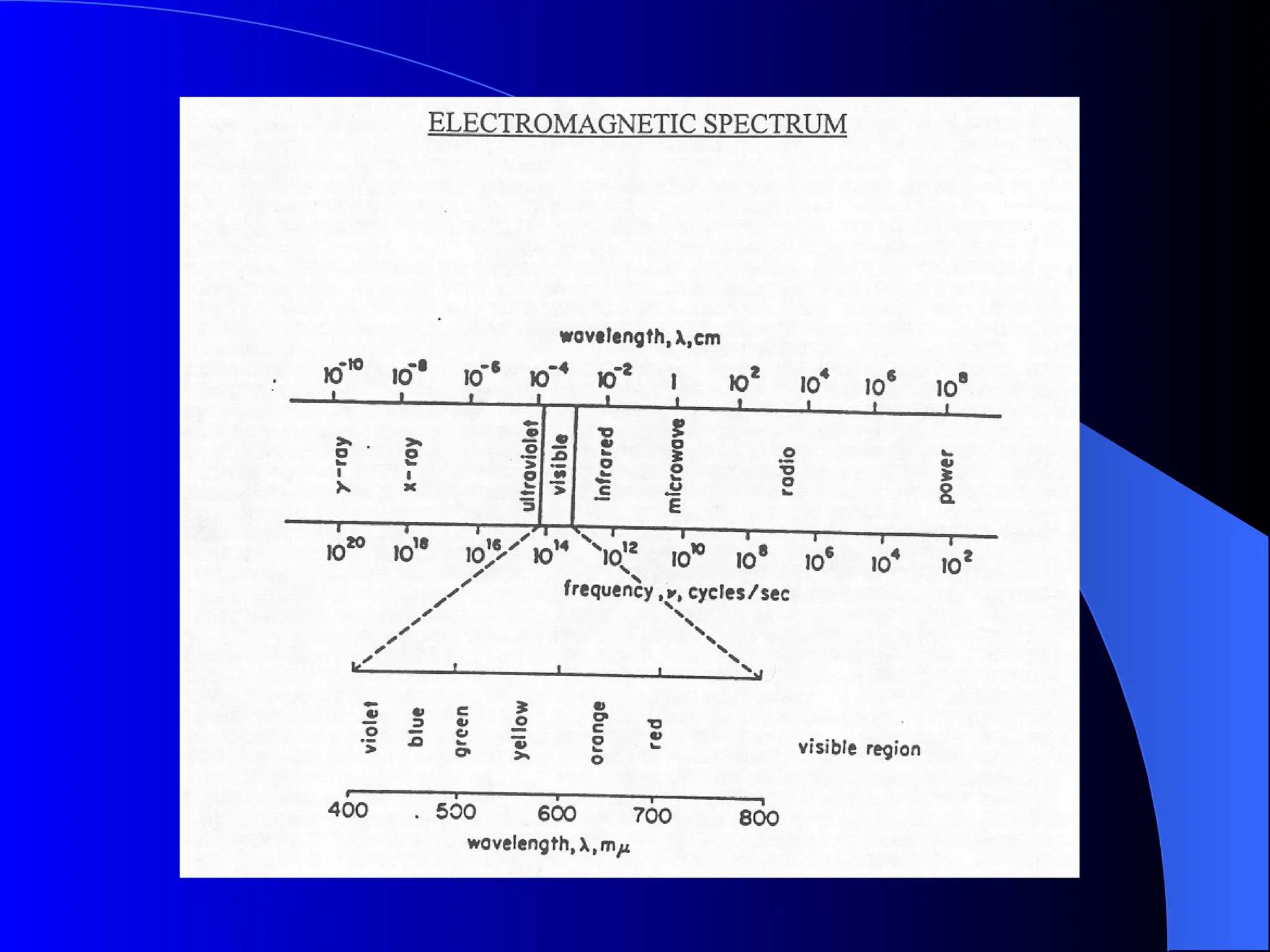
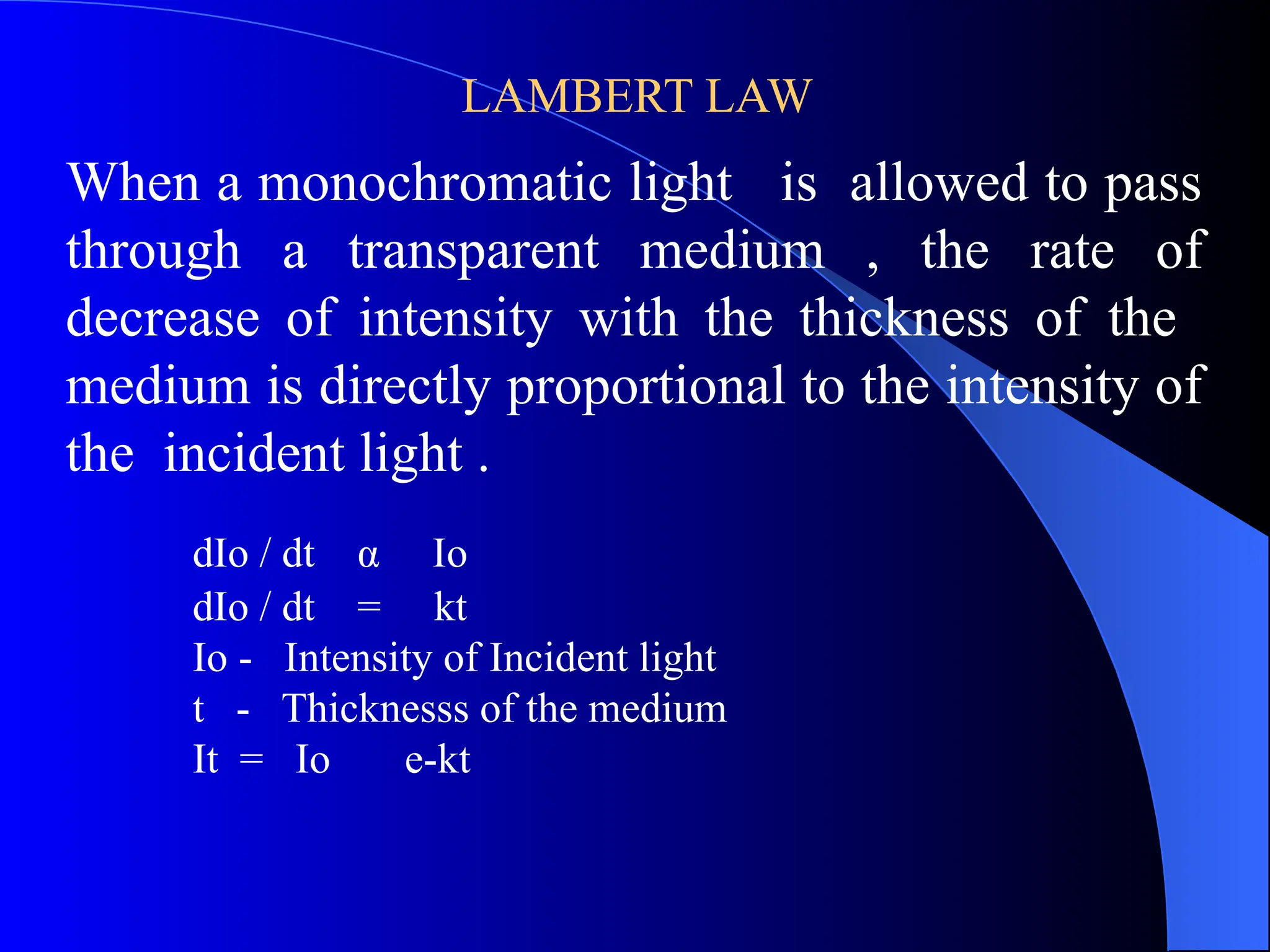
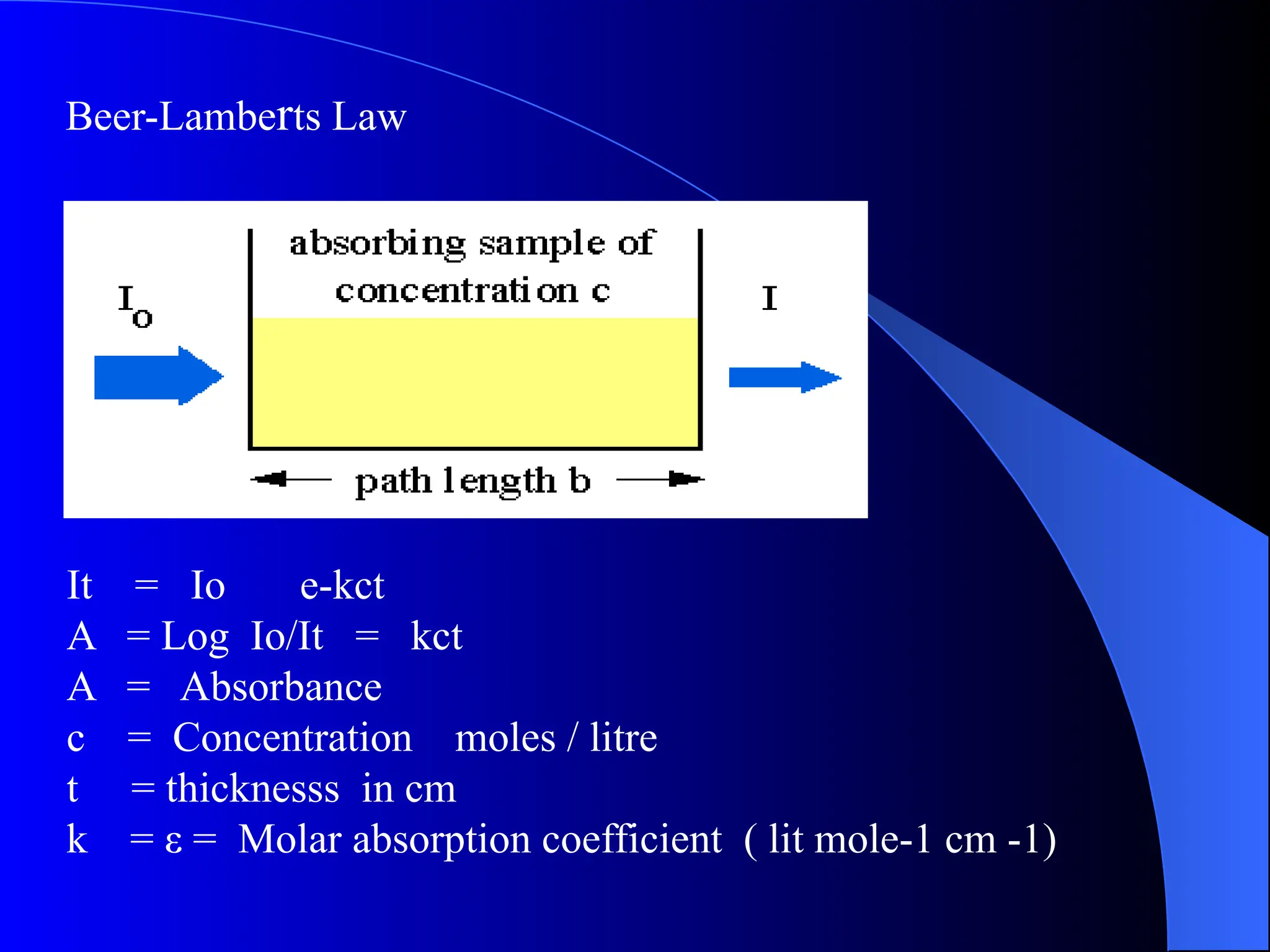
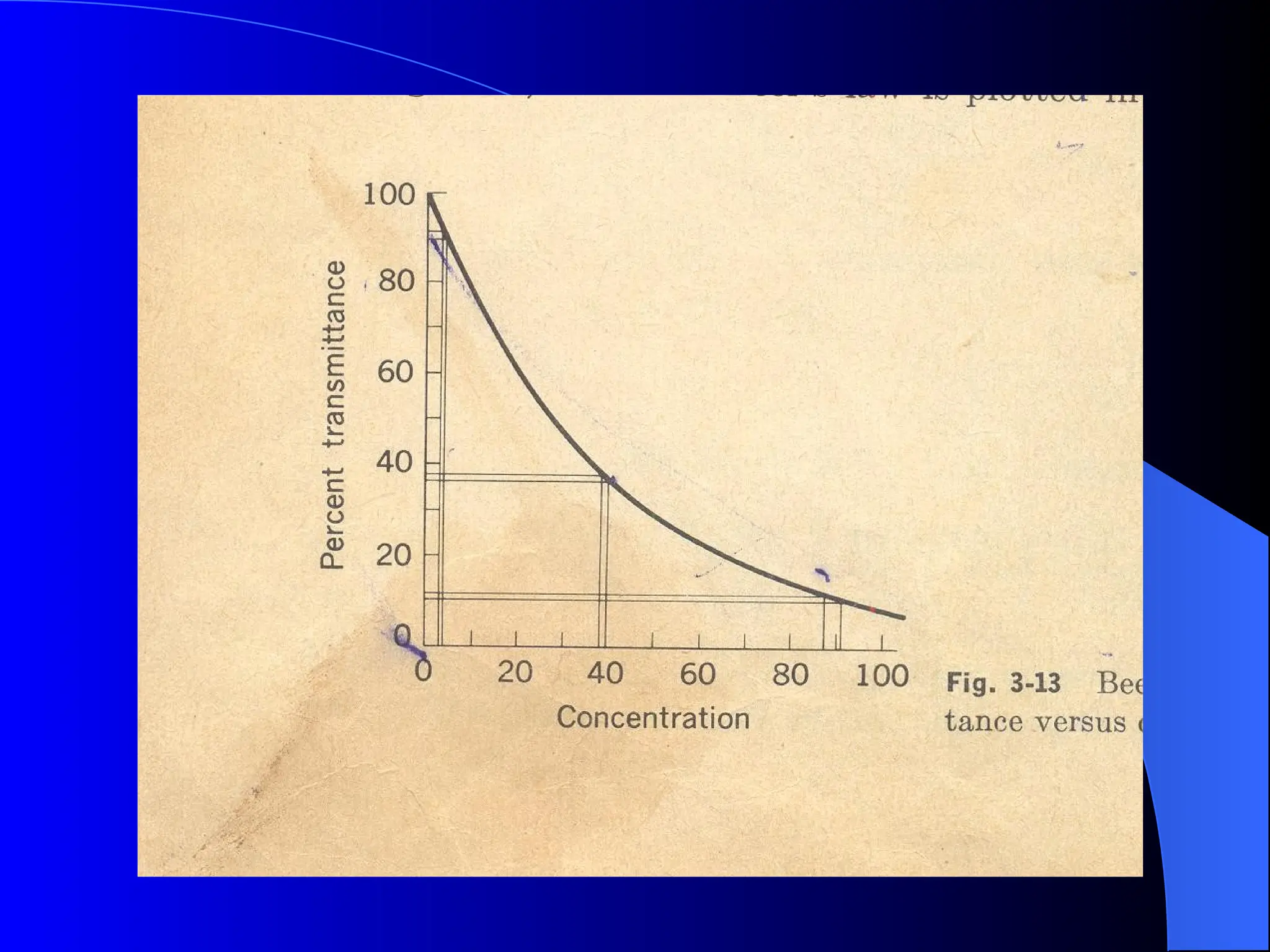
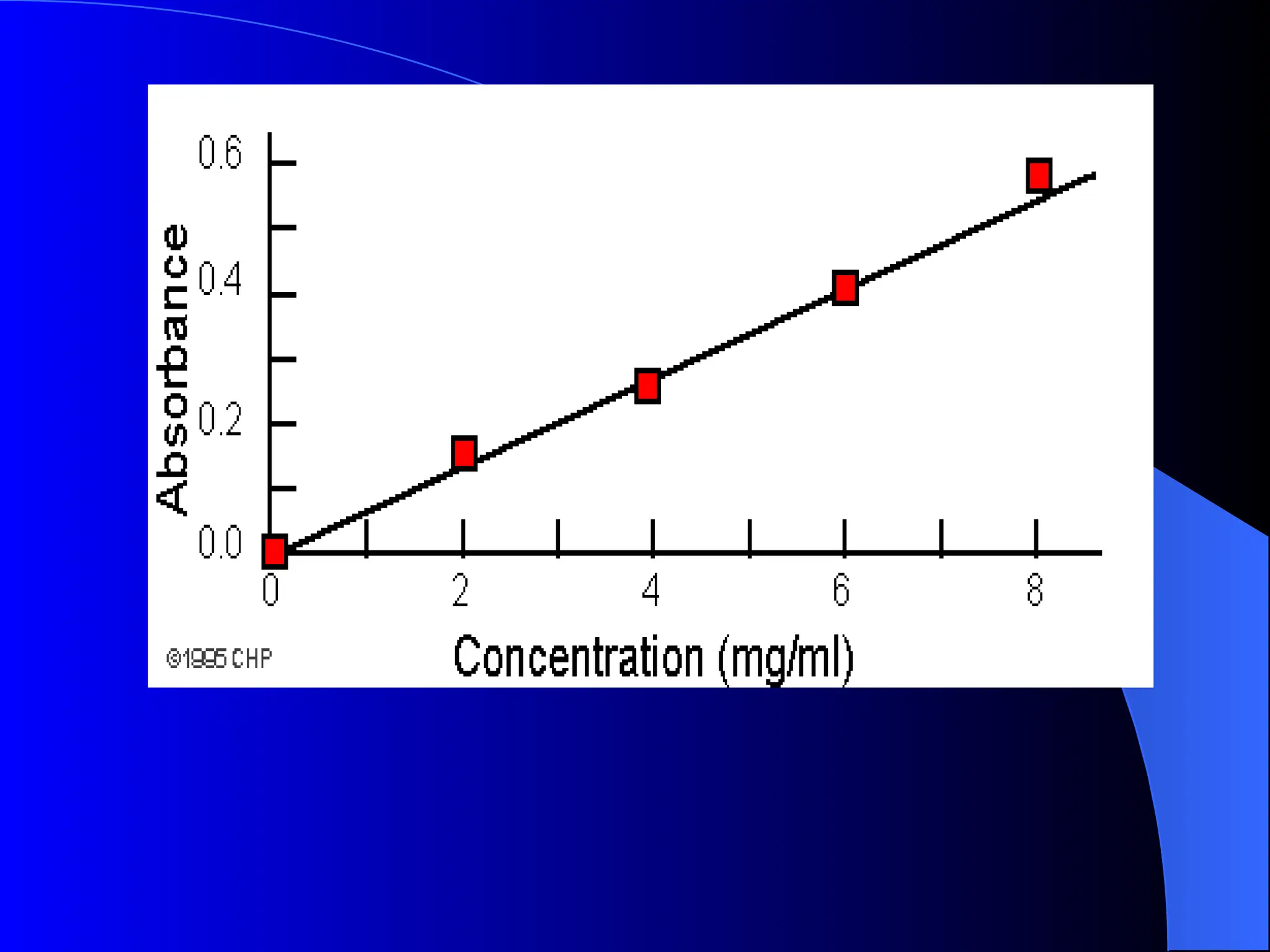
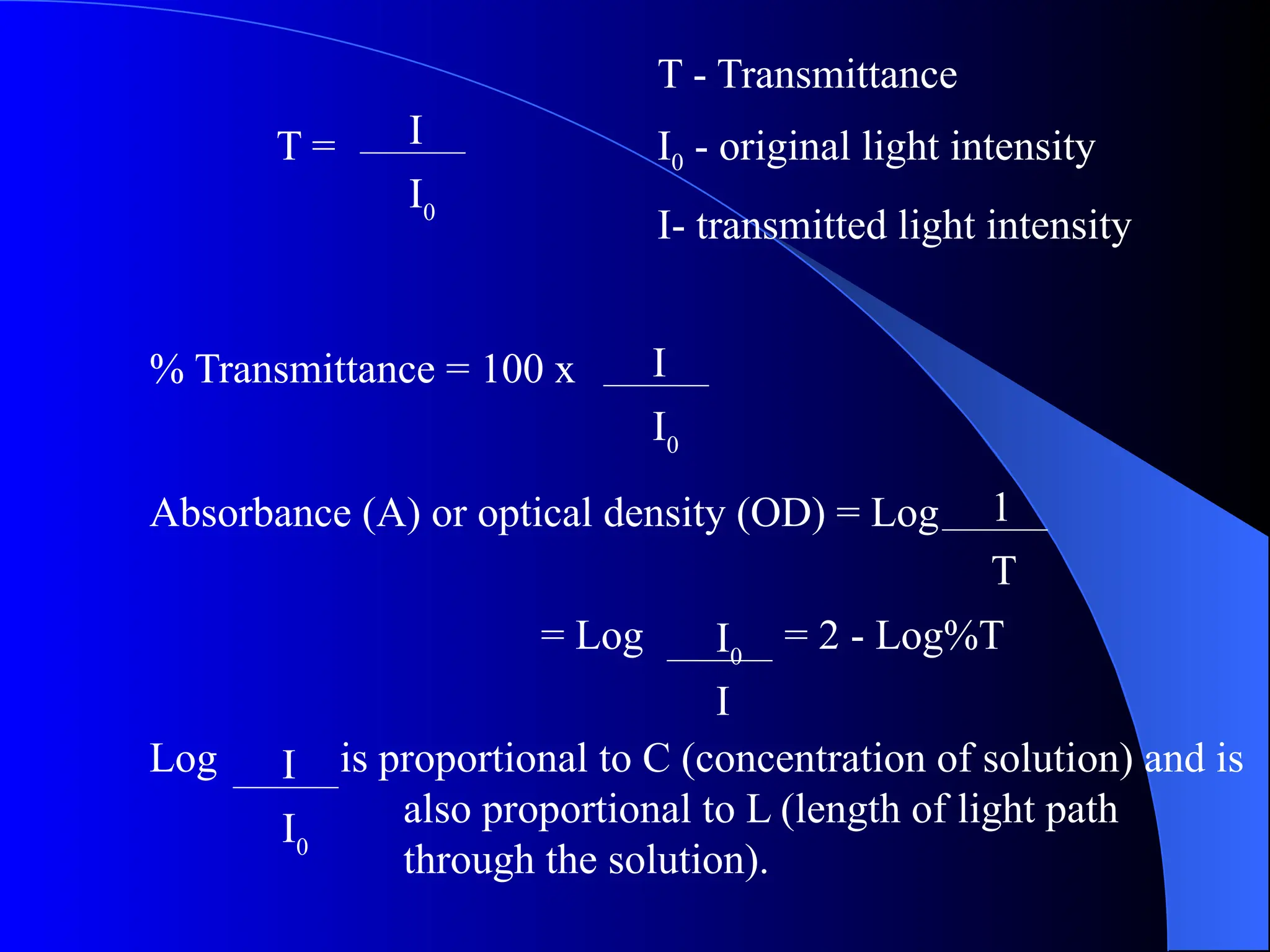
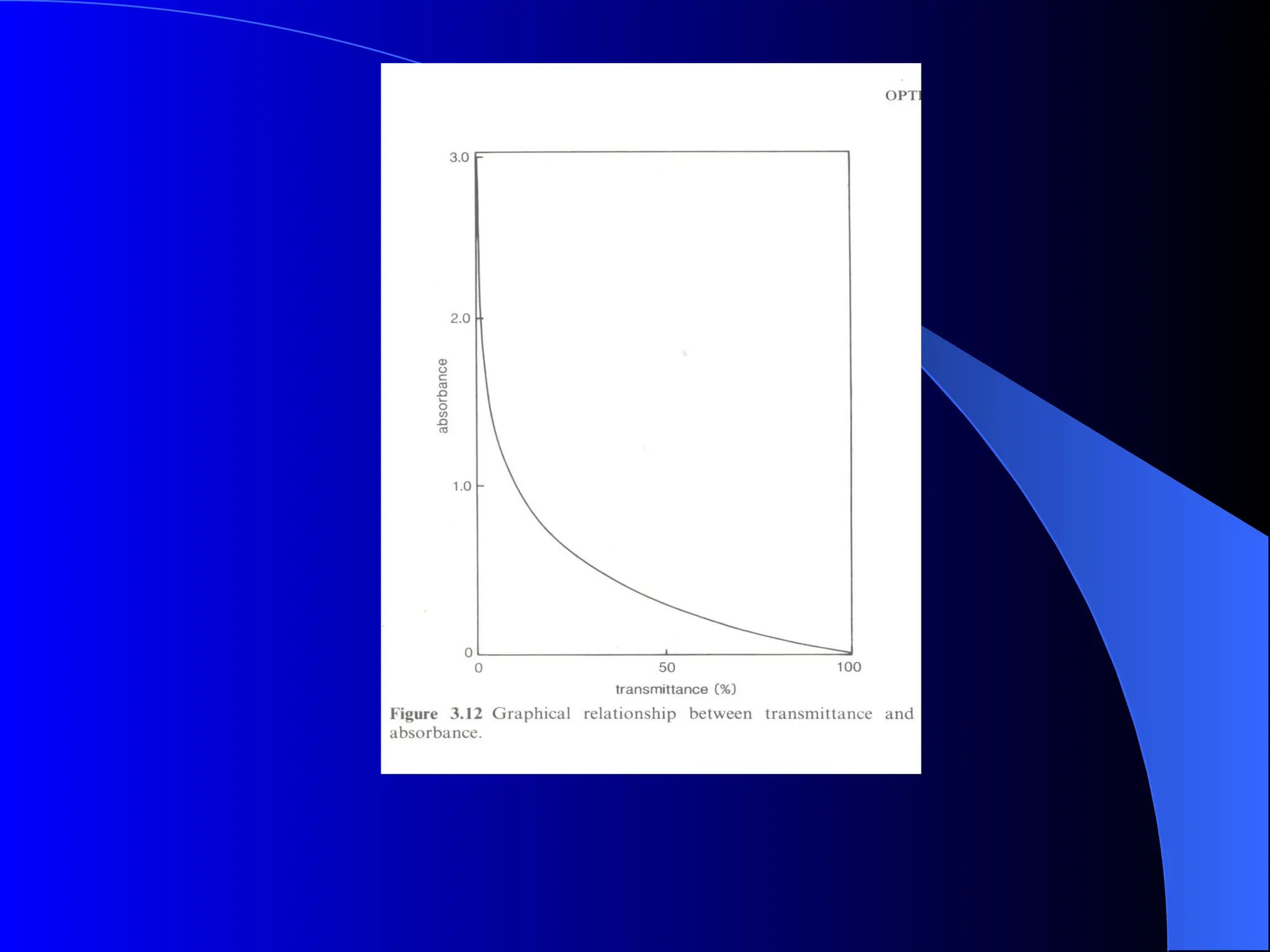
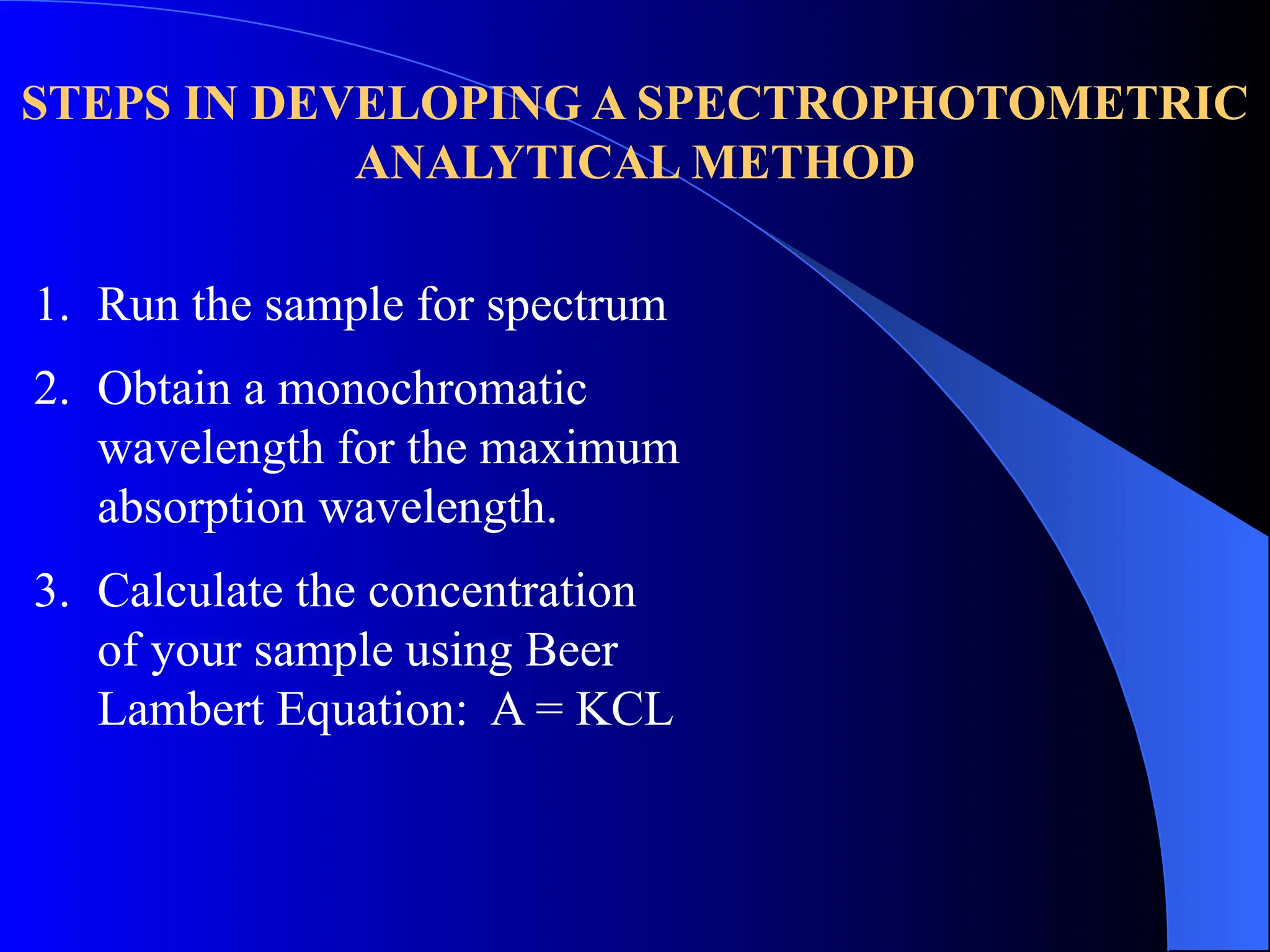
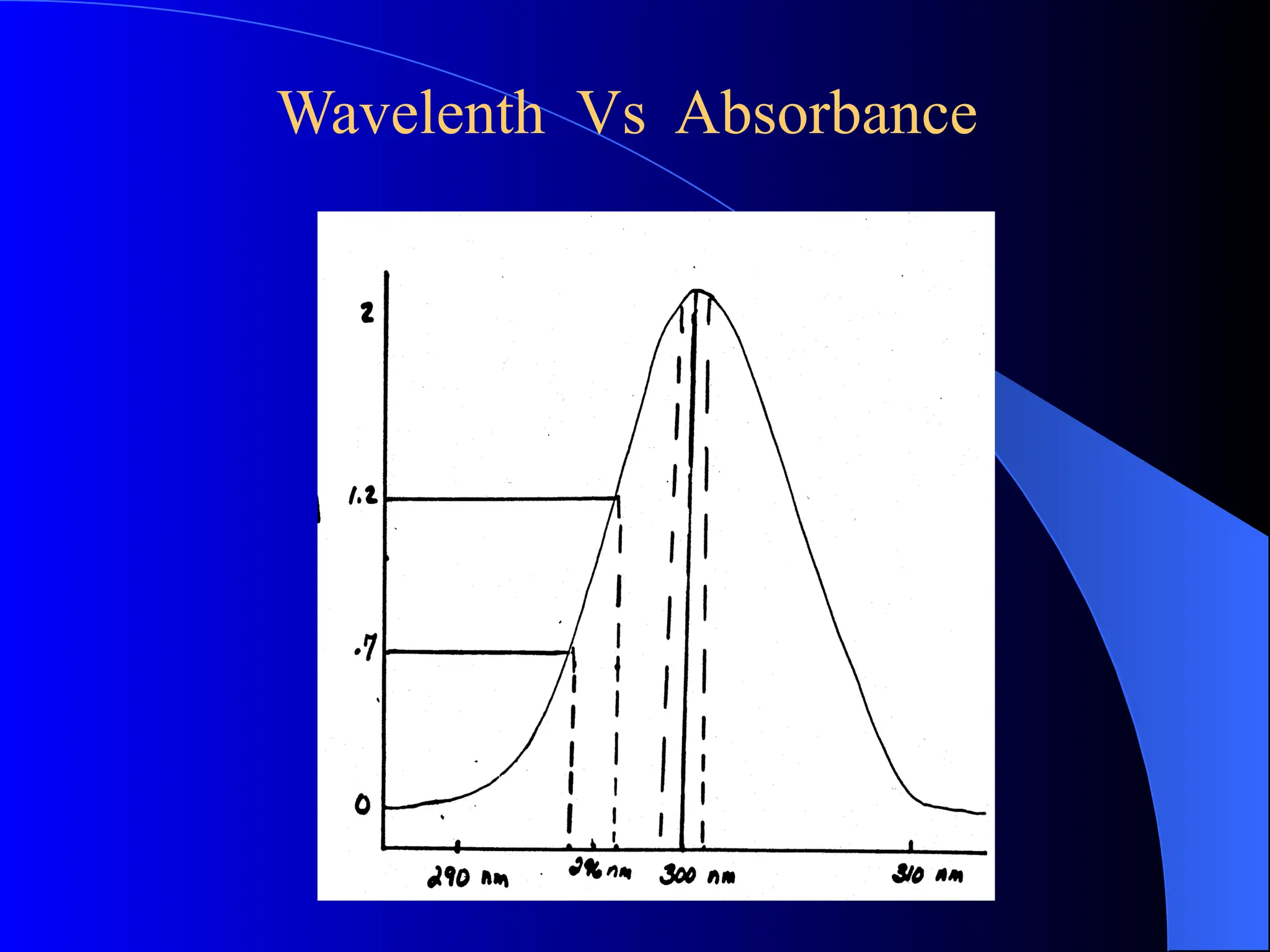
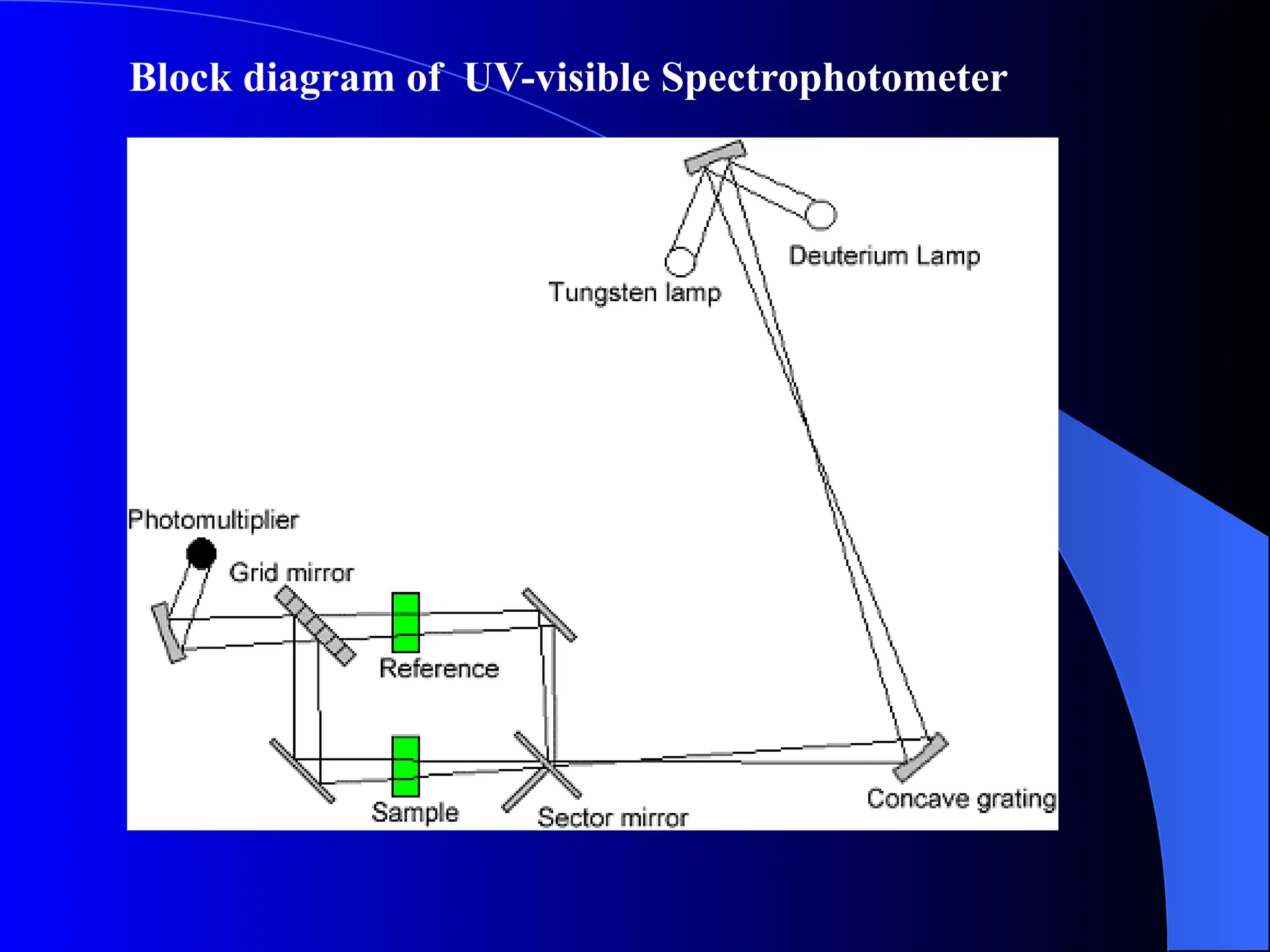
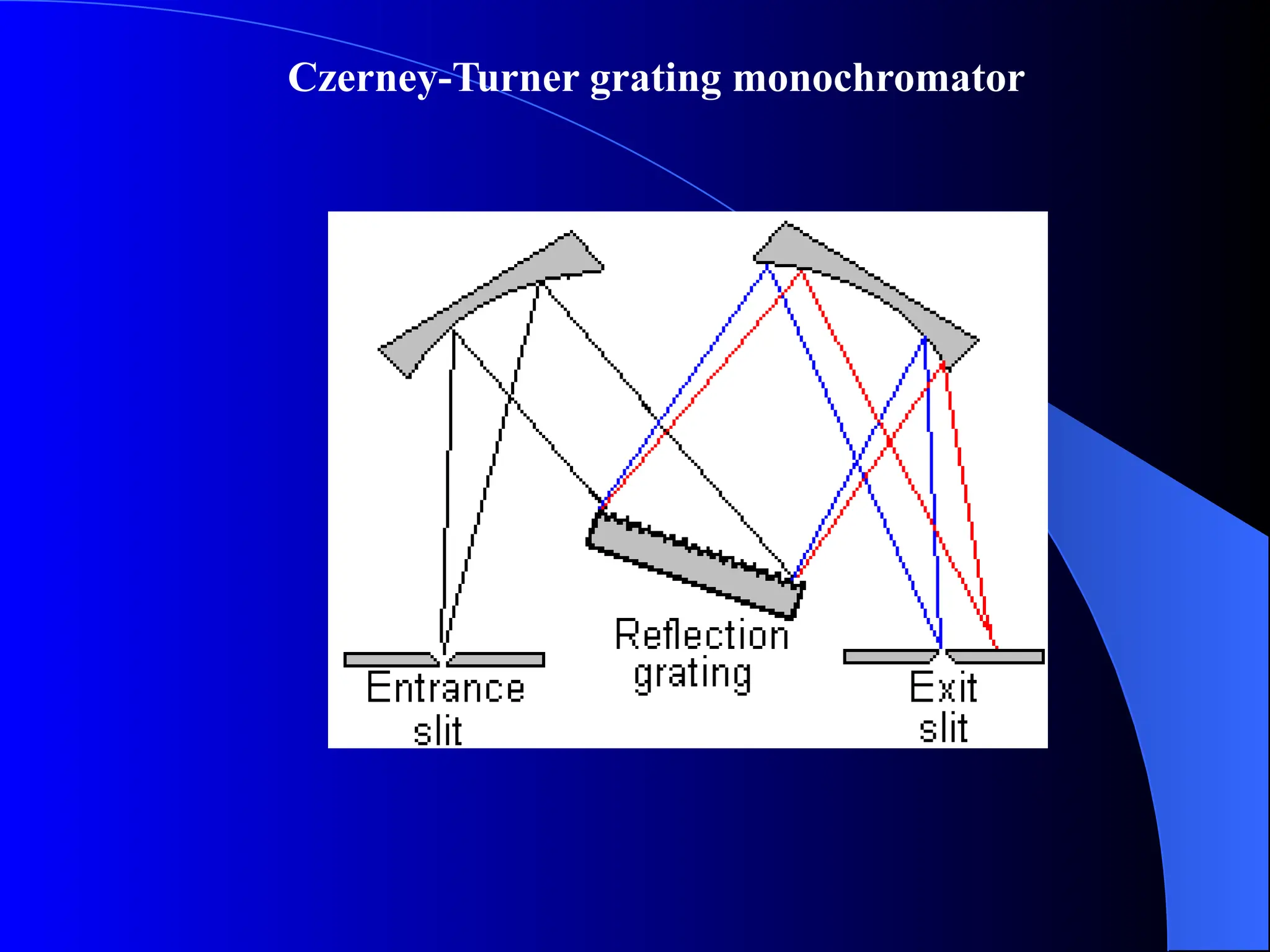
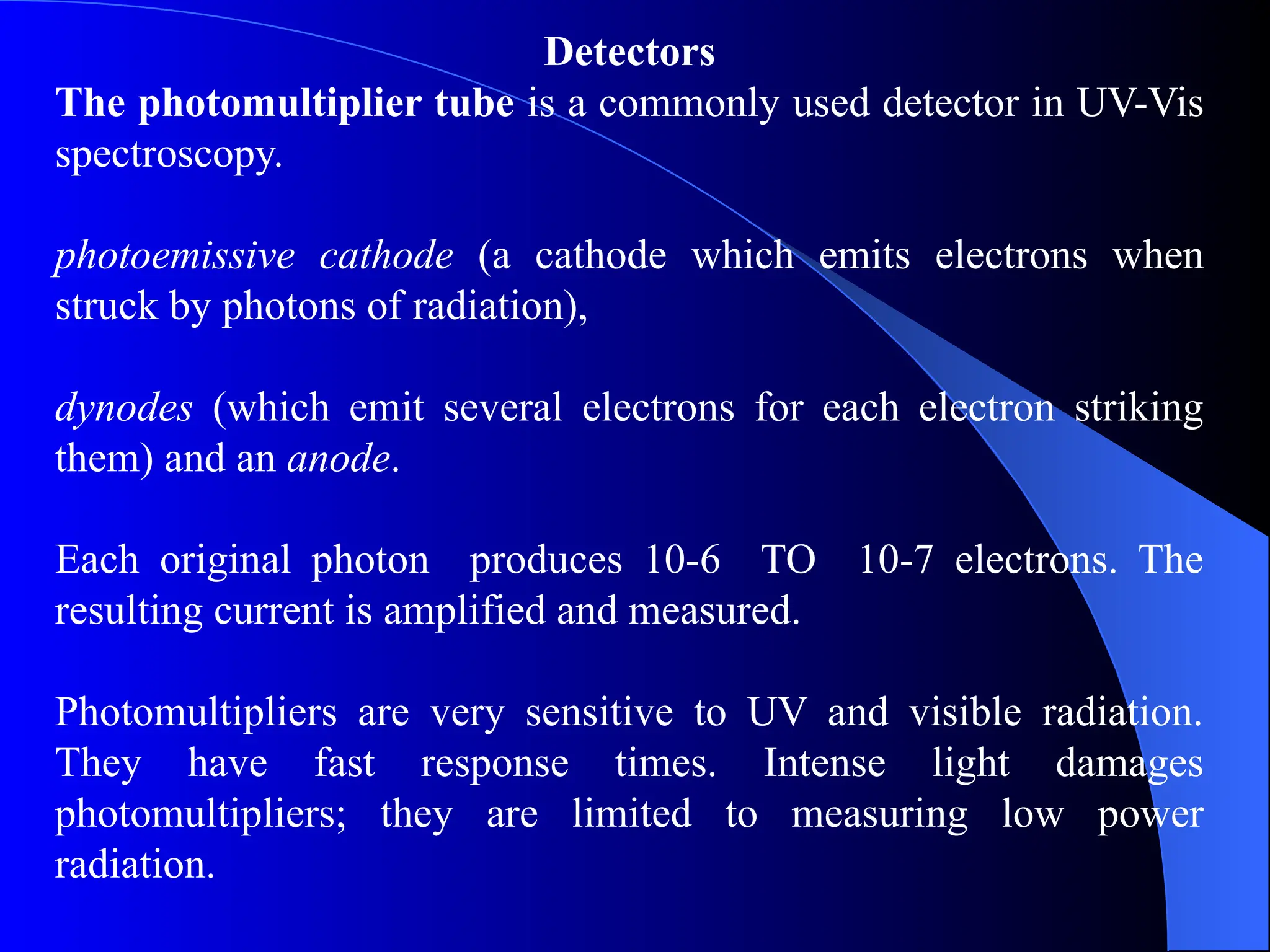
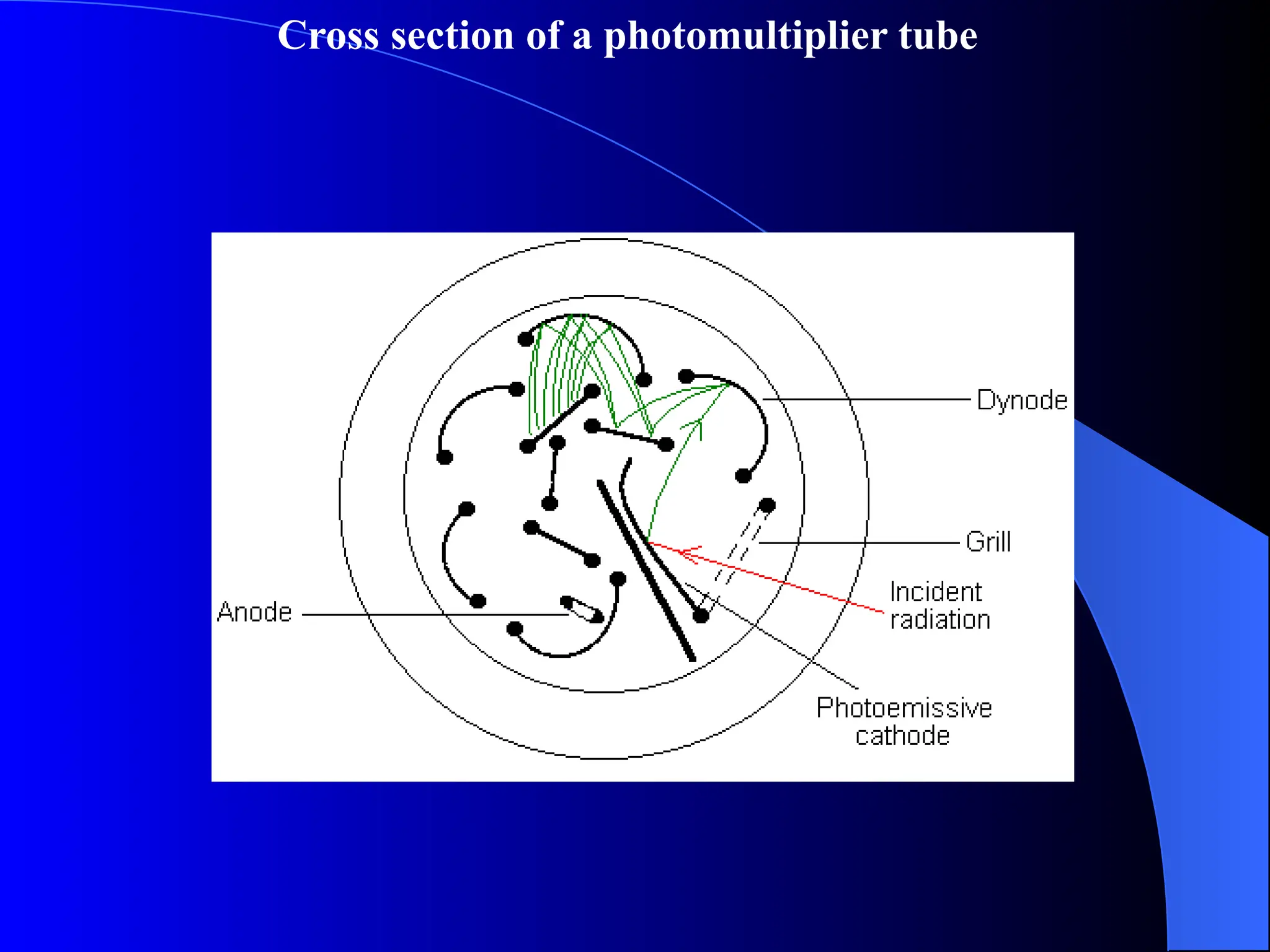
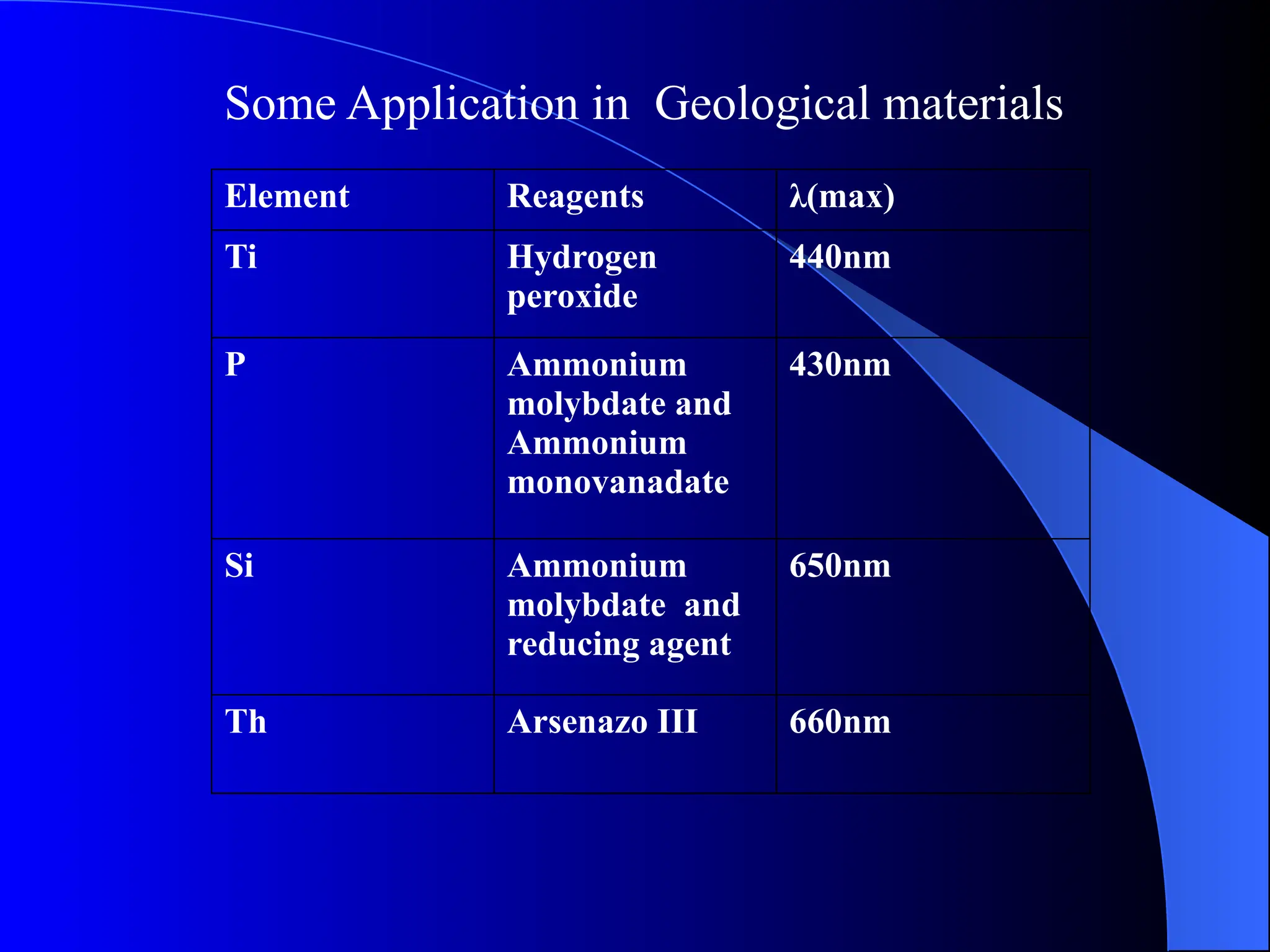
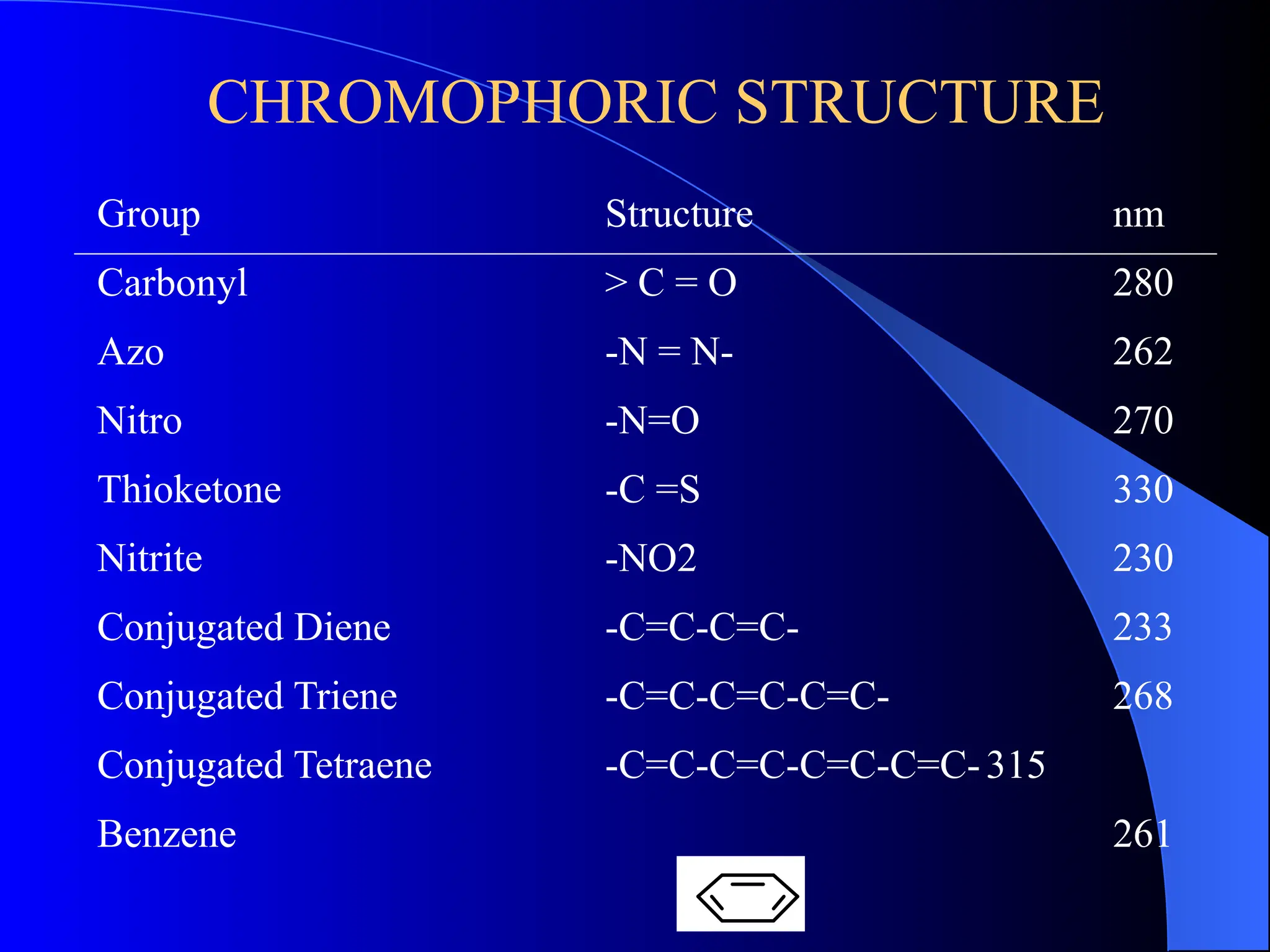
The document provides an overview of UV-visible molecular absorption spectrophotometry, discussing its theory, instrumentation, advantages, limitations, and applications. Key concepts include the Beer-Lambert law, sources of radiation, types of detectors, and the construction of spectrophotometric methods to analyze samples at trace levels. Specific applications are mentioned, including the determination of proteins, amino acids, and various metals.