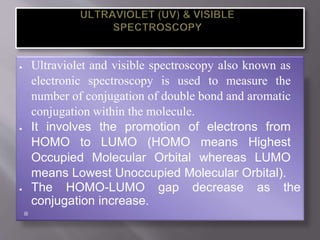
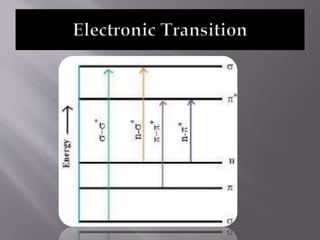
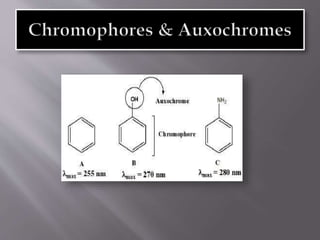
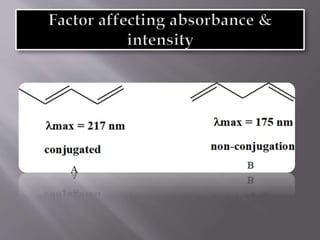
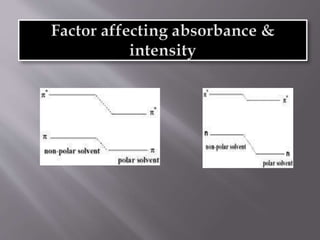
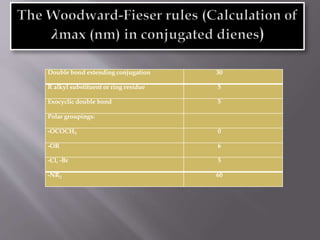
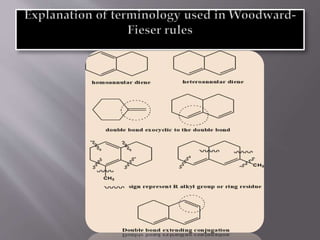
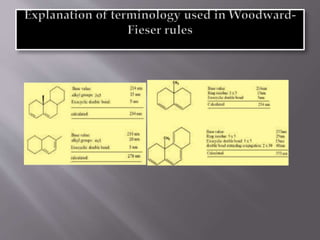
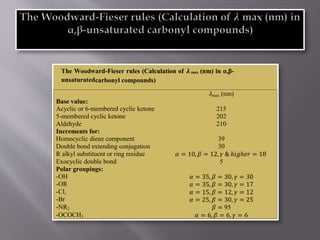
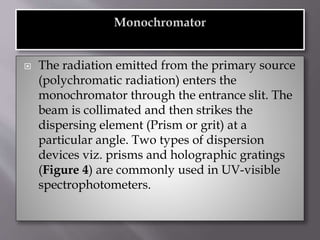
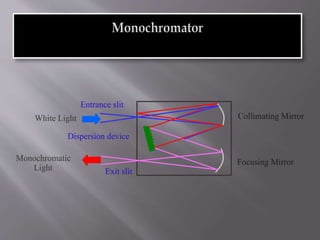
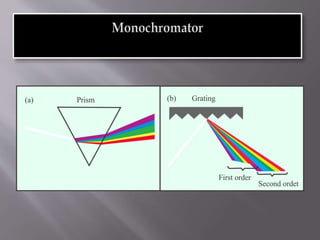
Ultraviolet-visible spectroscopy involves promoting electrons from the highest occupied molecular orbital to the lowest unoccupied molecular orbital when molecules absorb electromagnetic radiation. This causes different types of electronic transitions that can be observed based on wavelength shifts. The technique follows Beer's law where absorbance is directly proportional to concentration and path length. It is used to determine conjugation and identify functional groups in molecules.