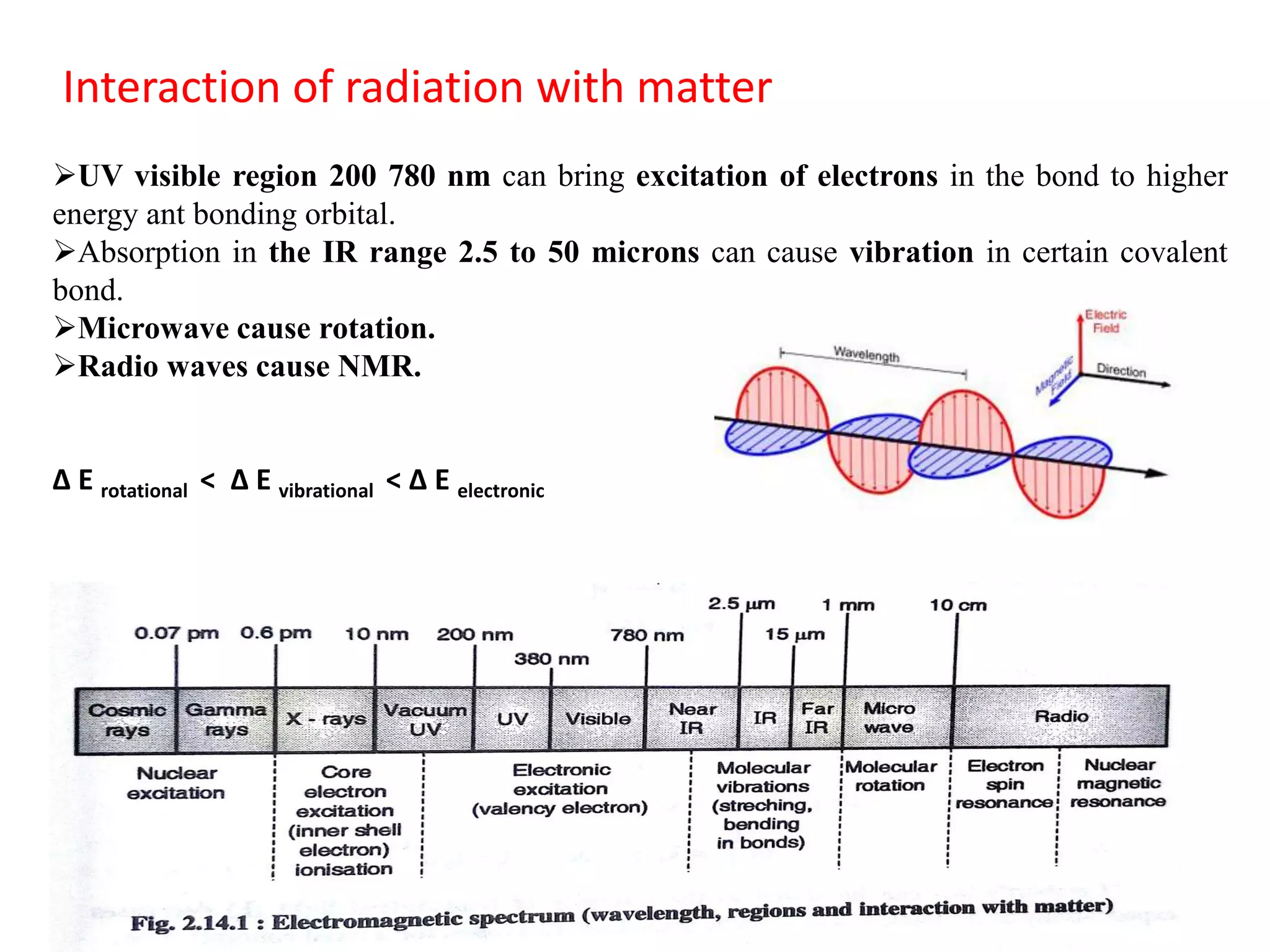
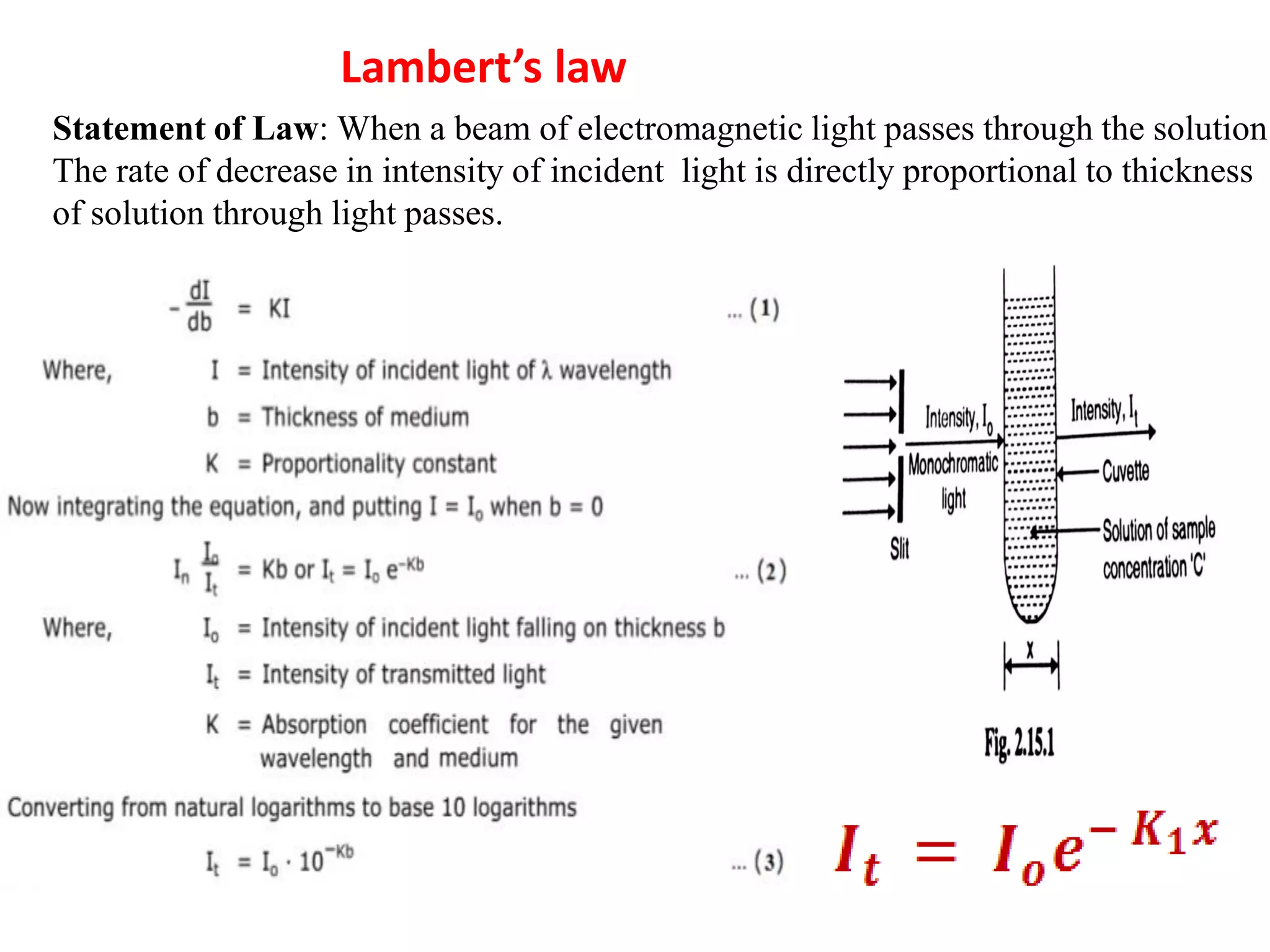
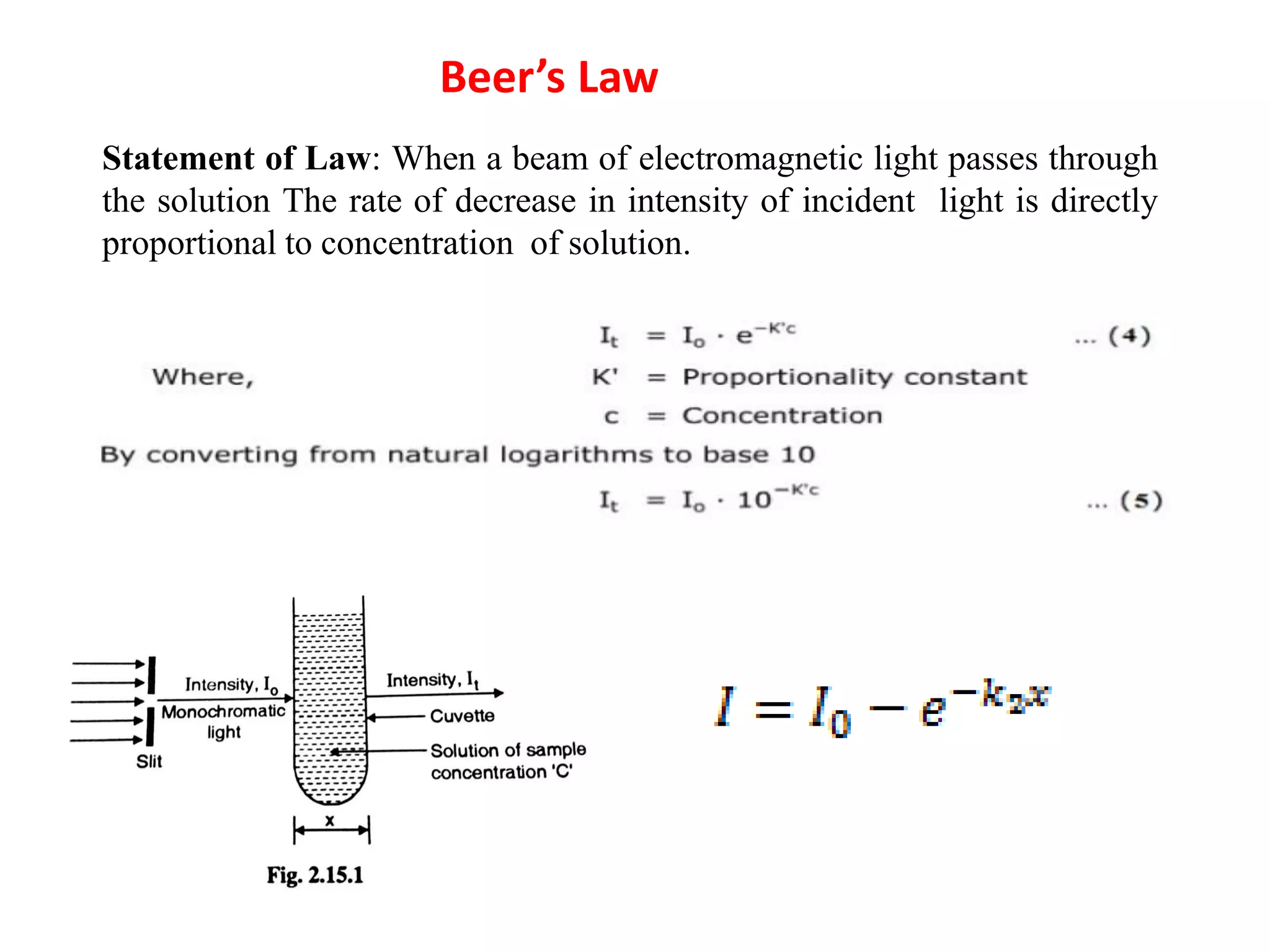
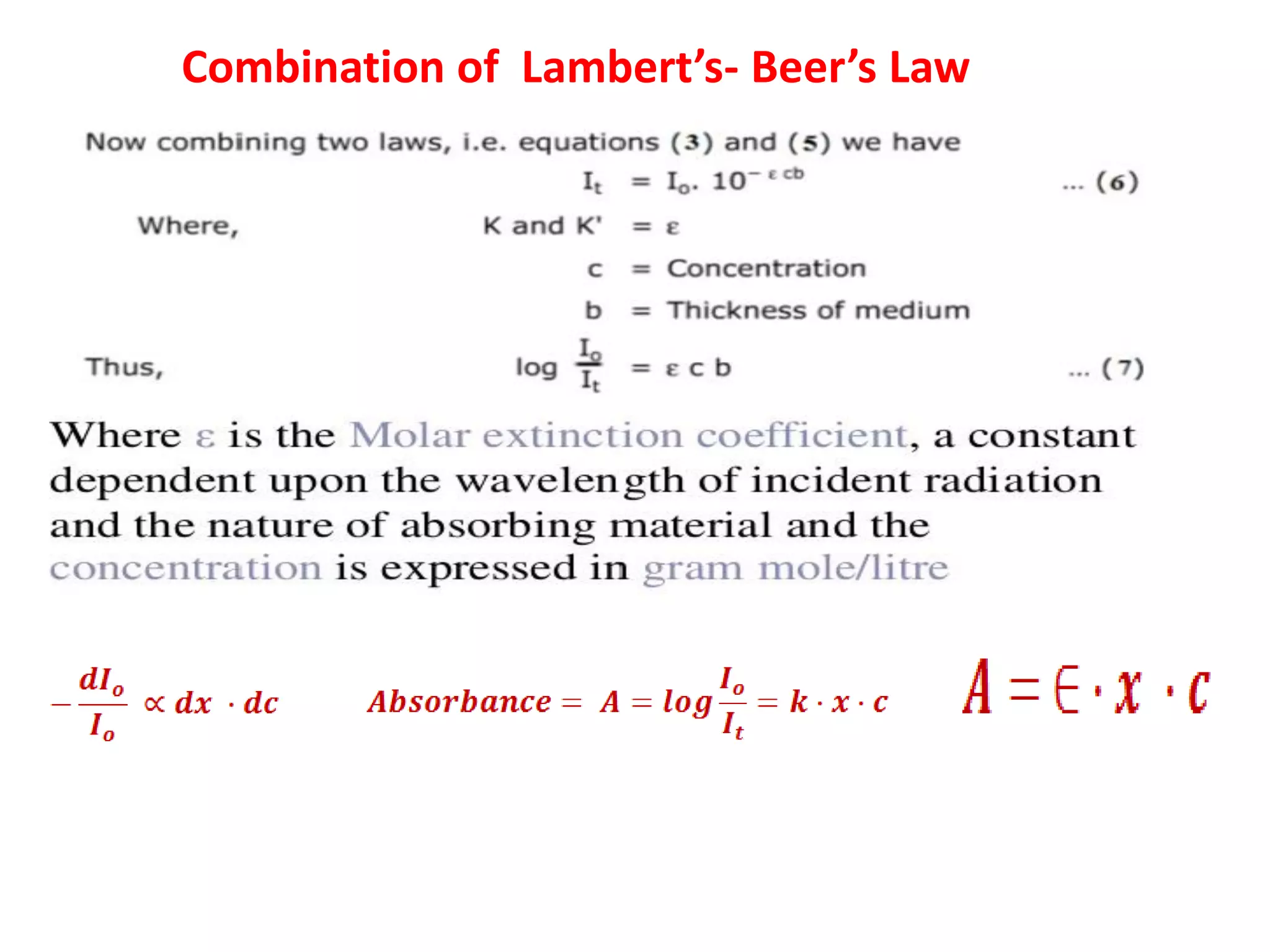
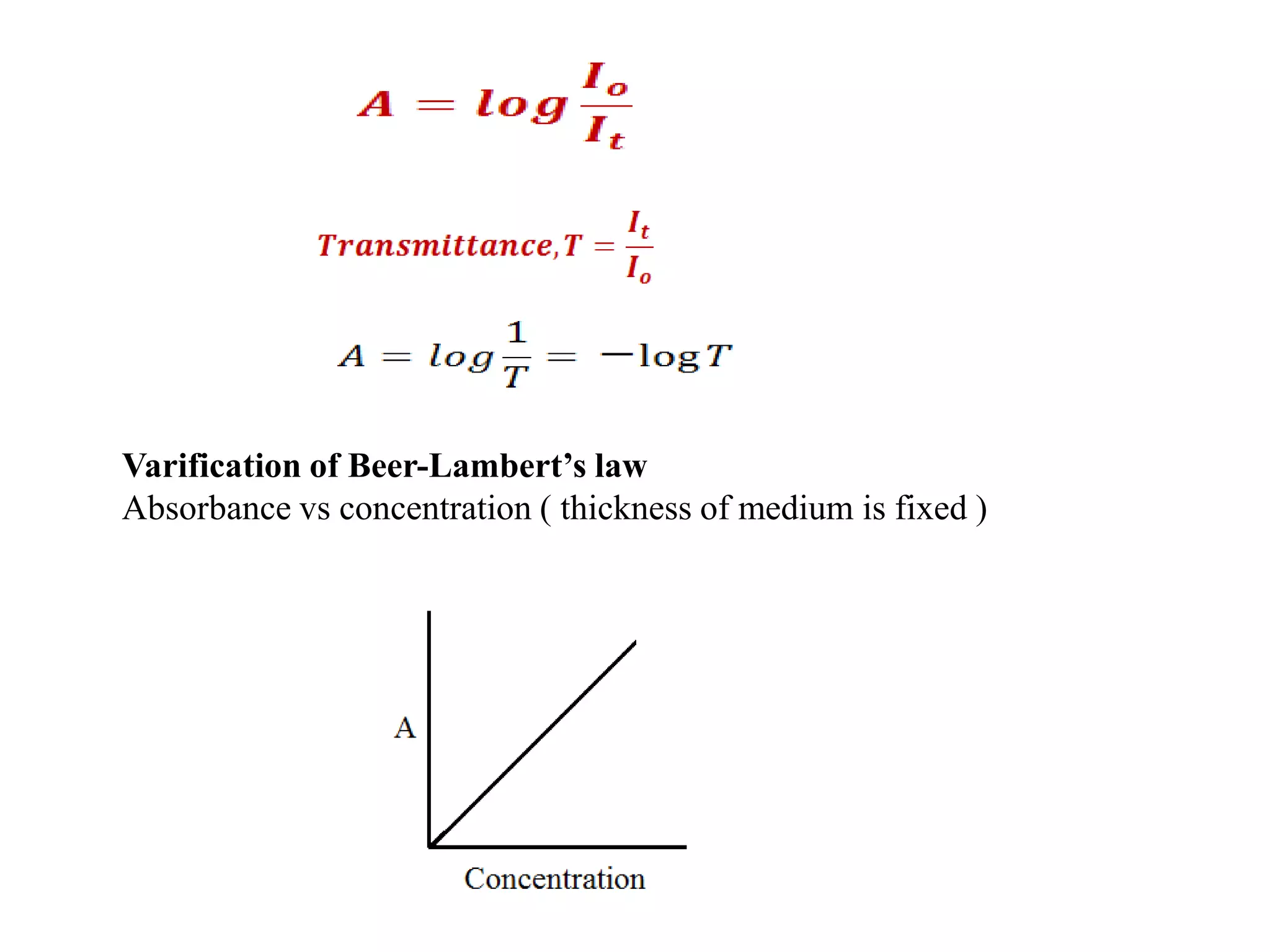
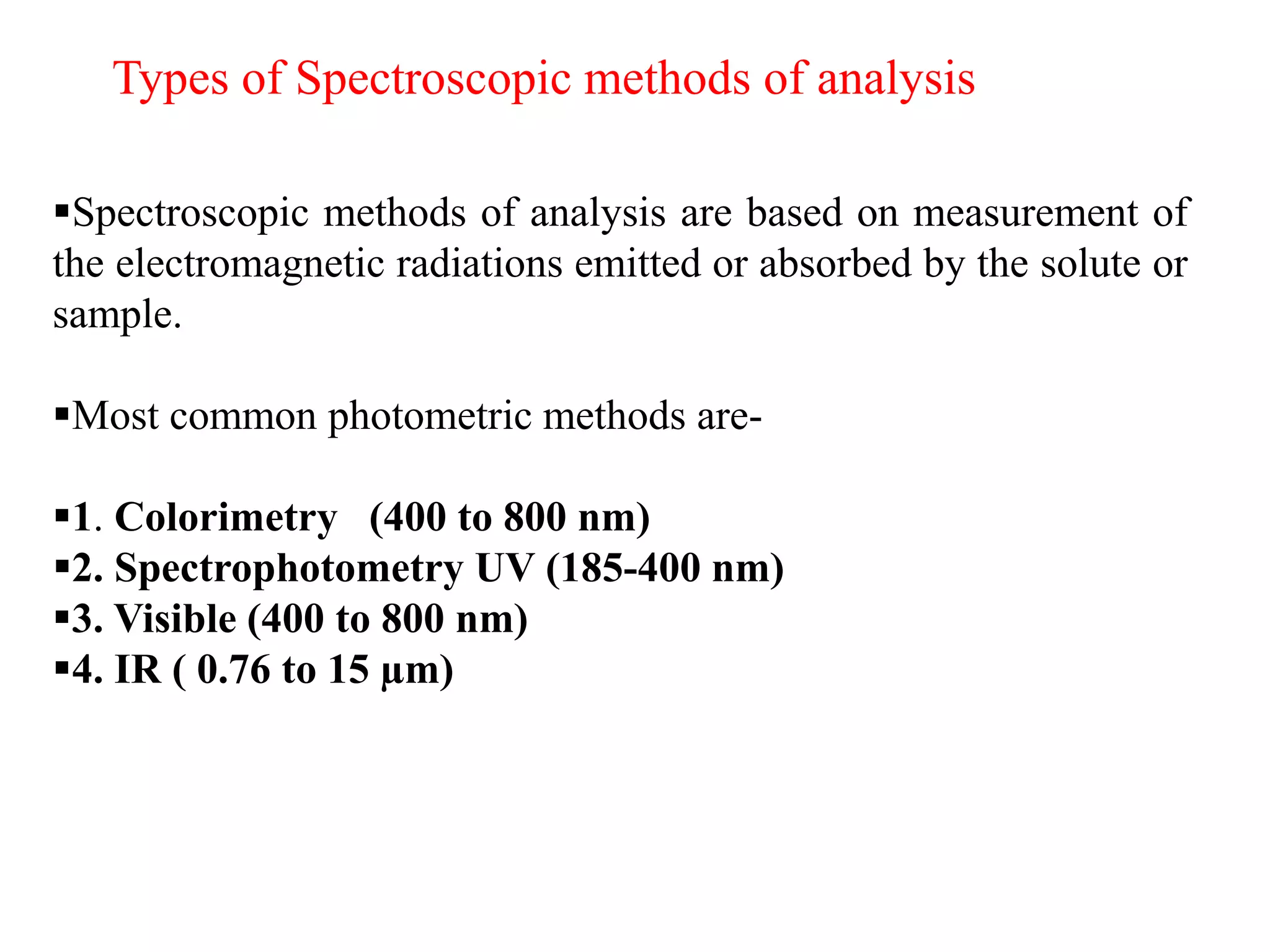
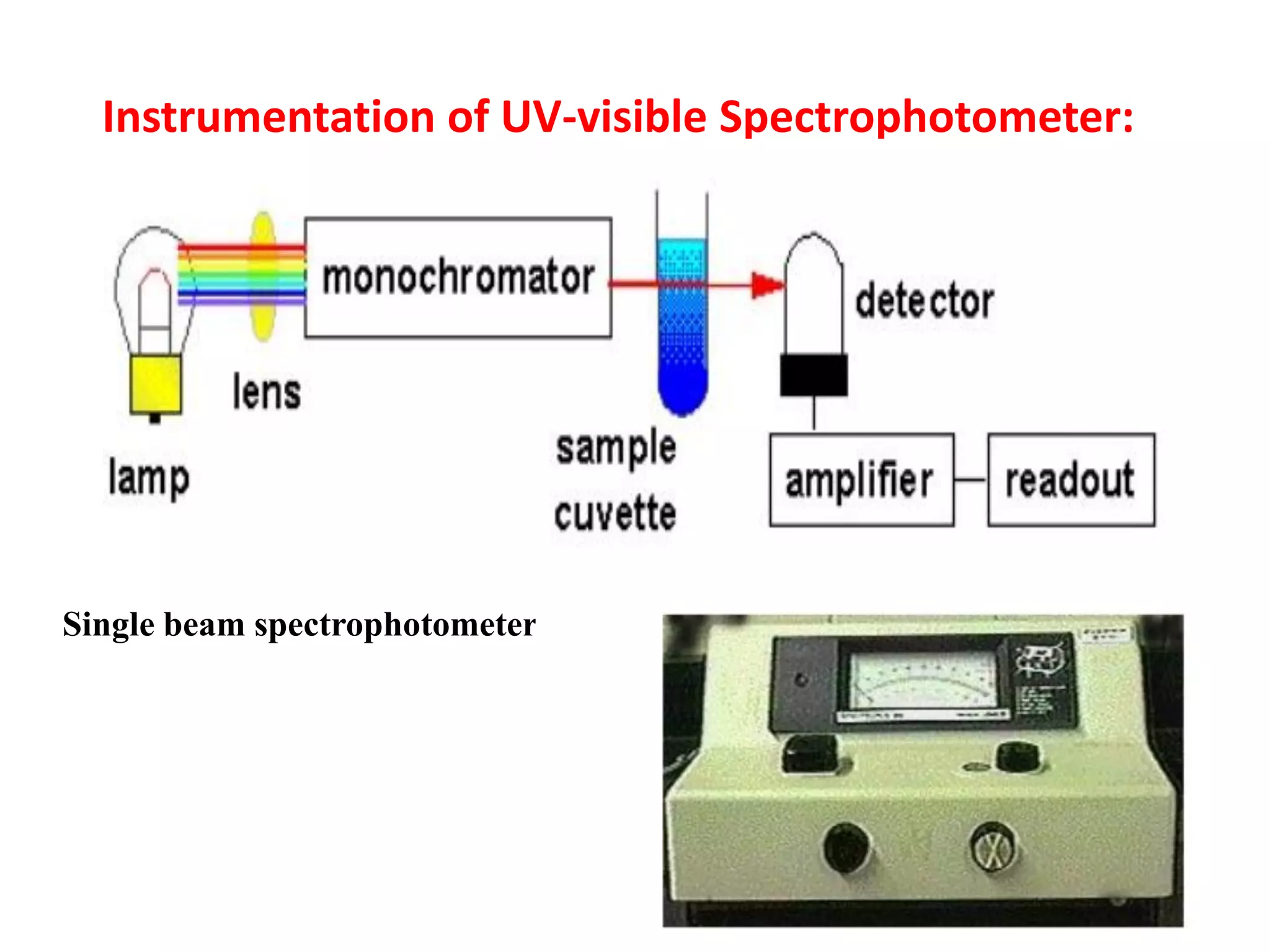
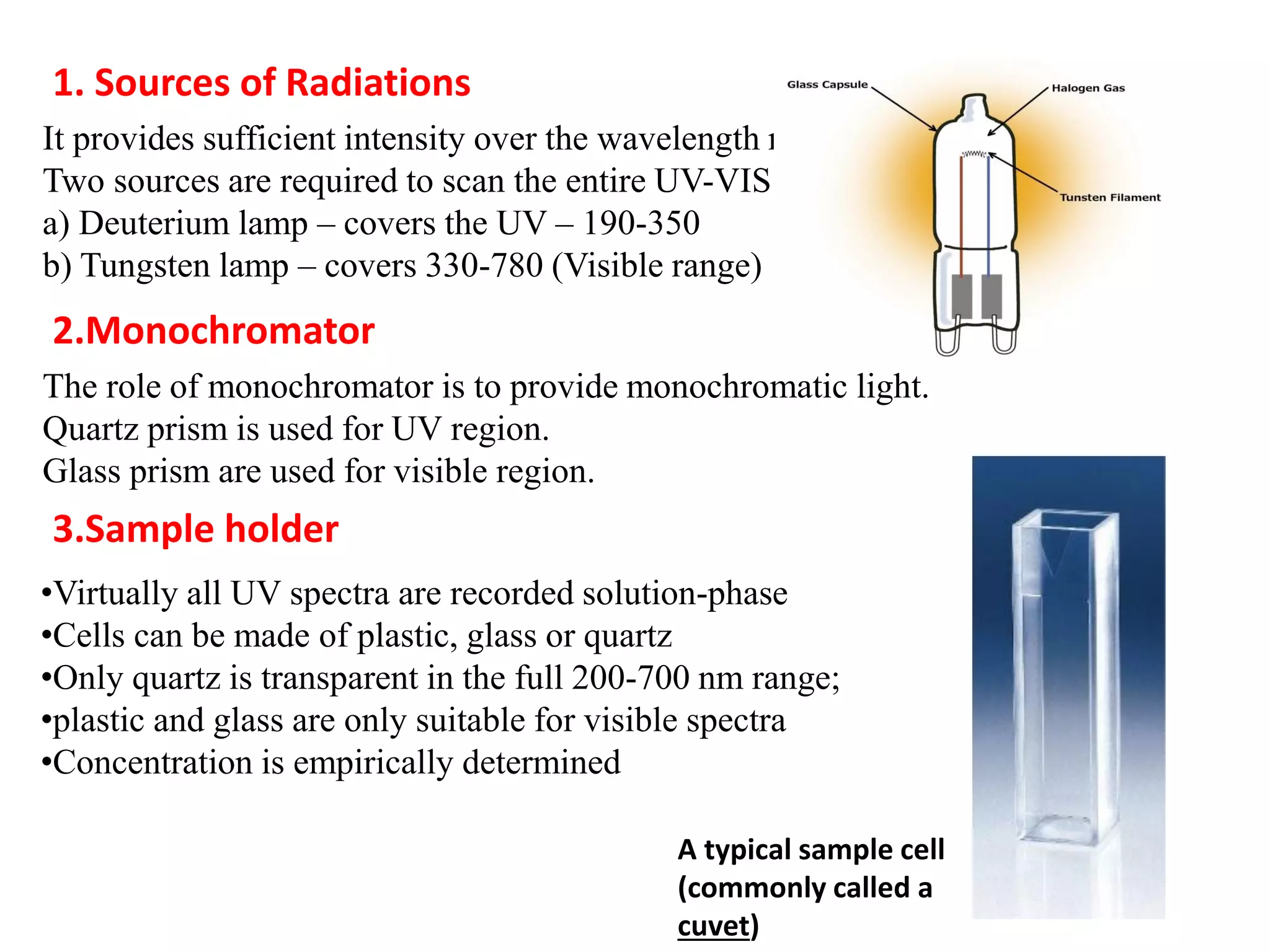
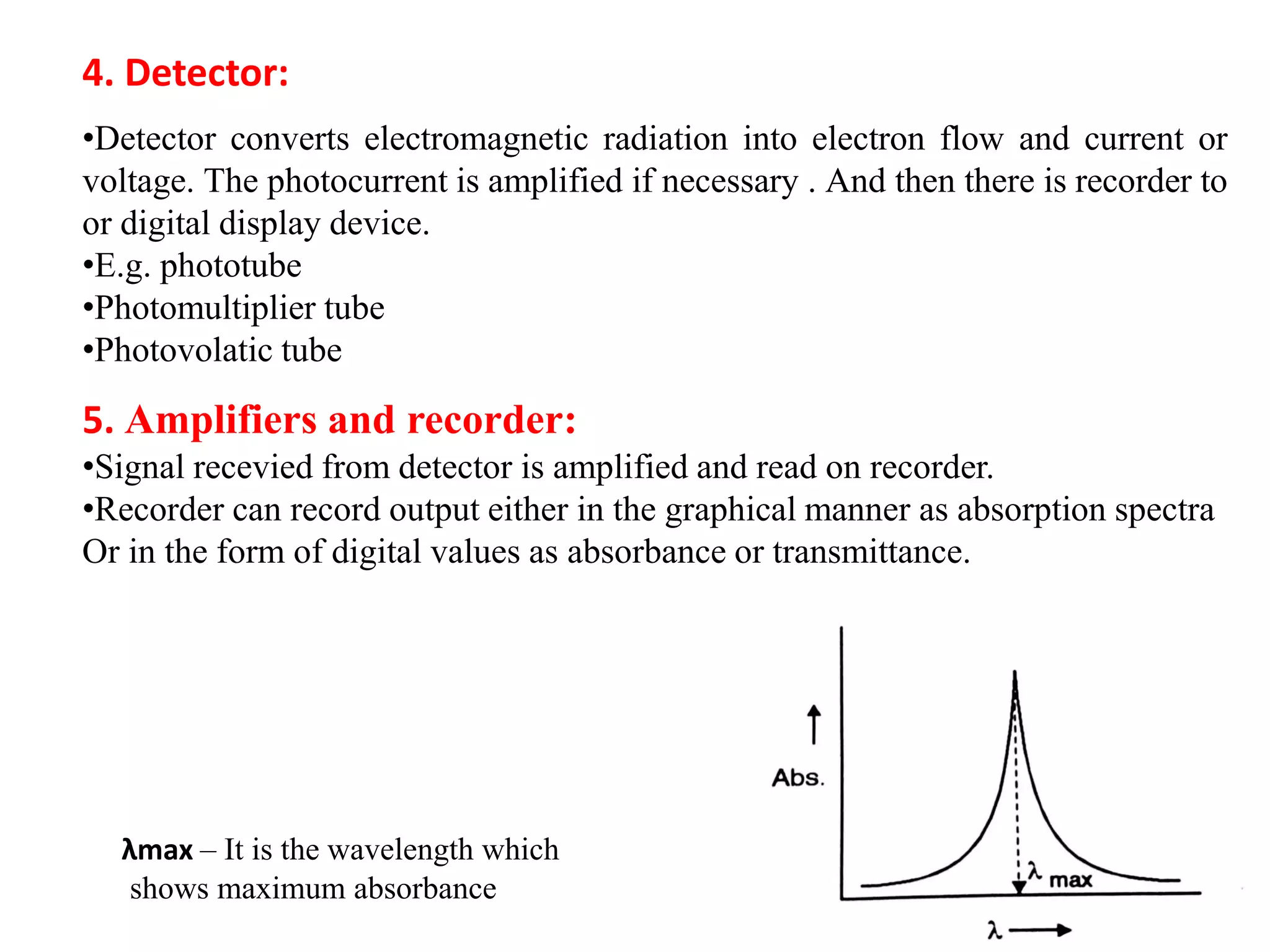
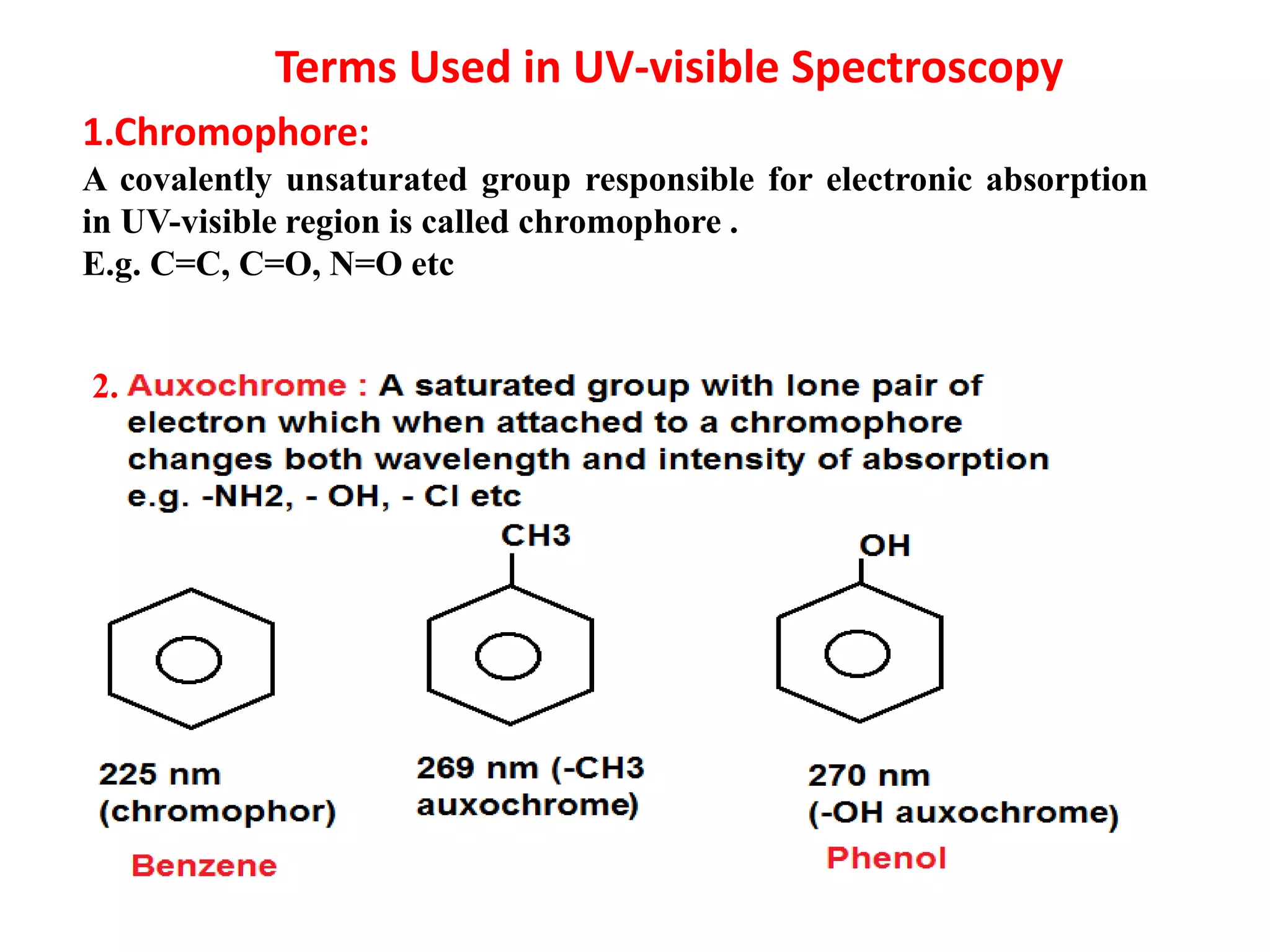
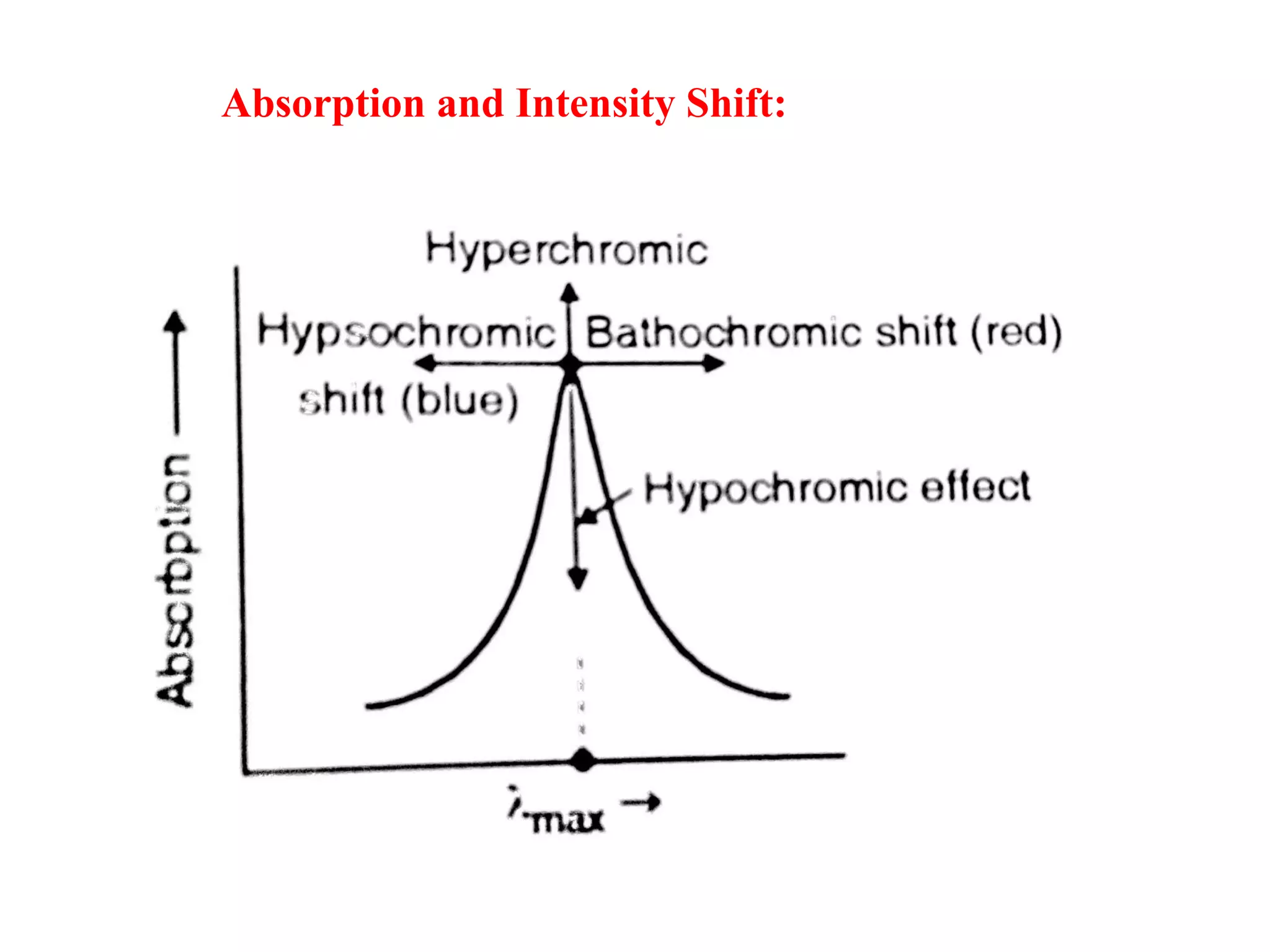
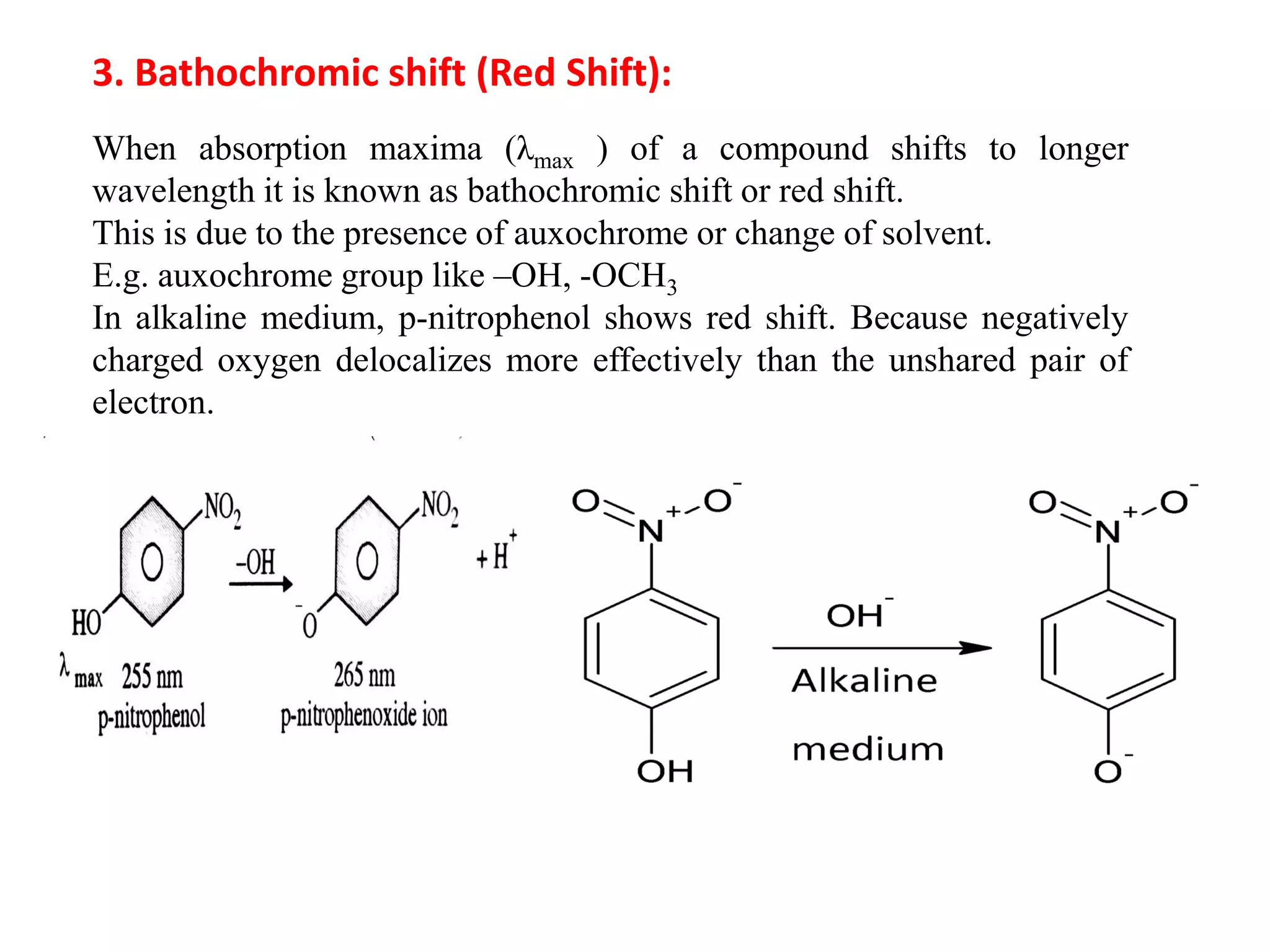
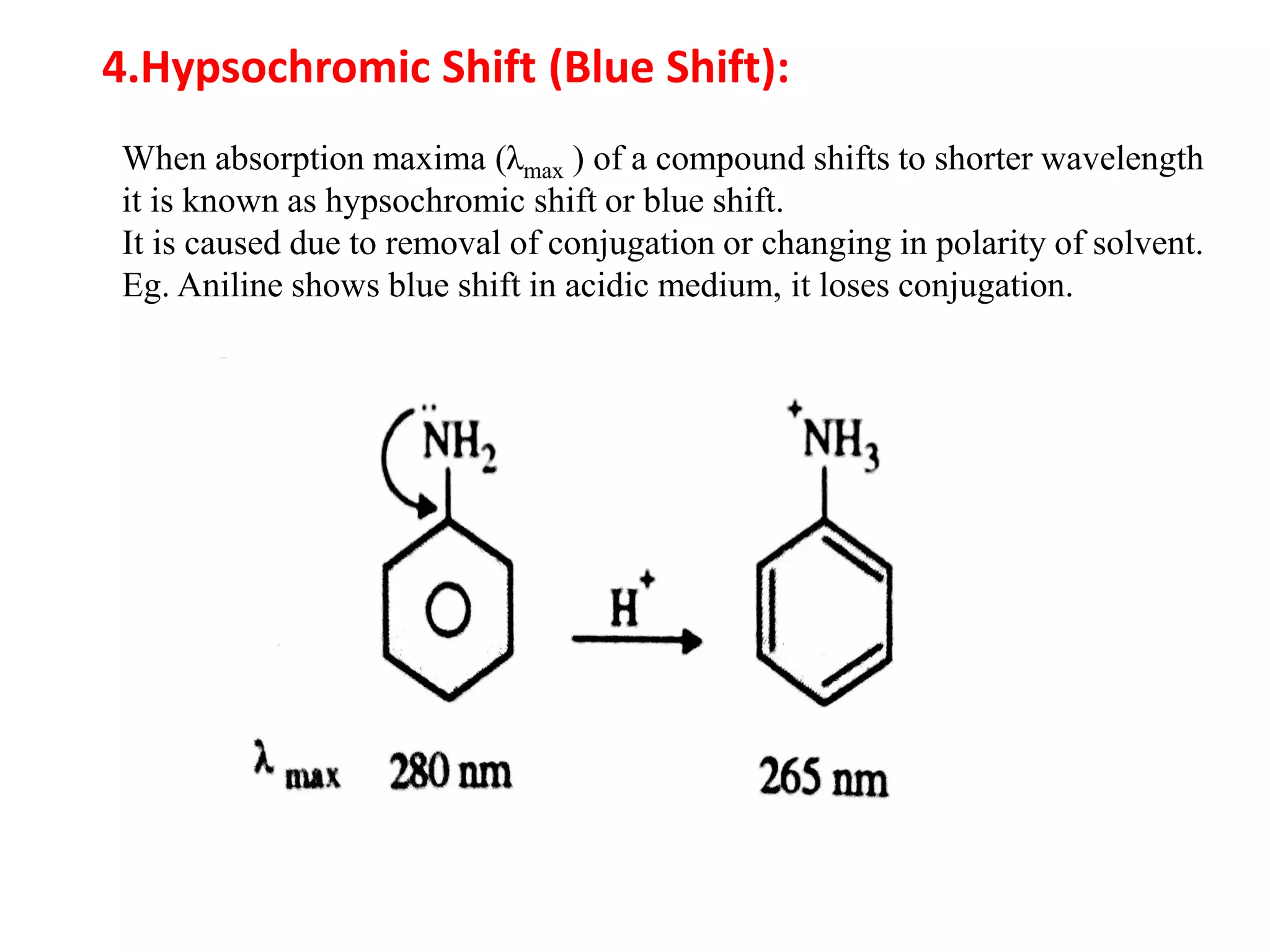
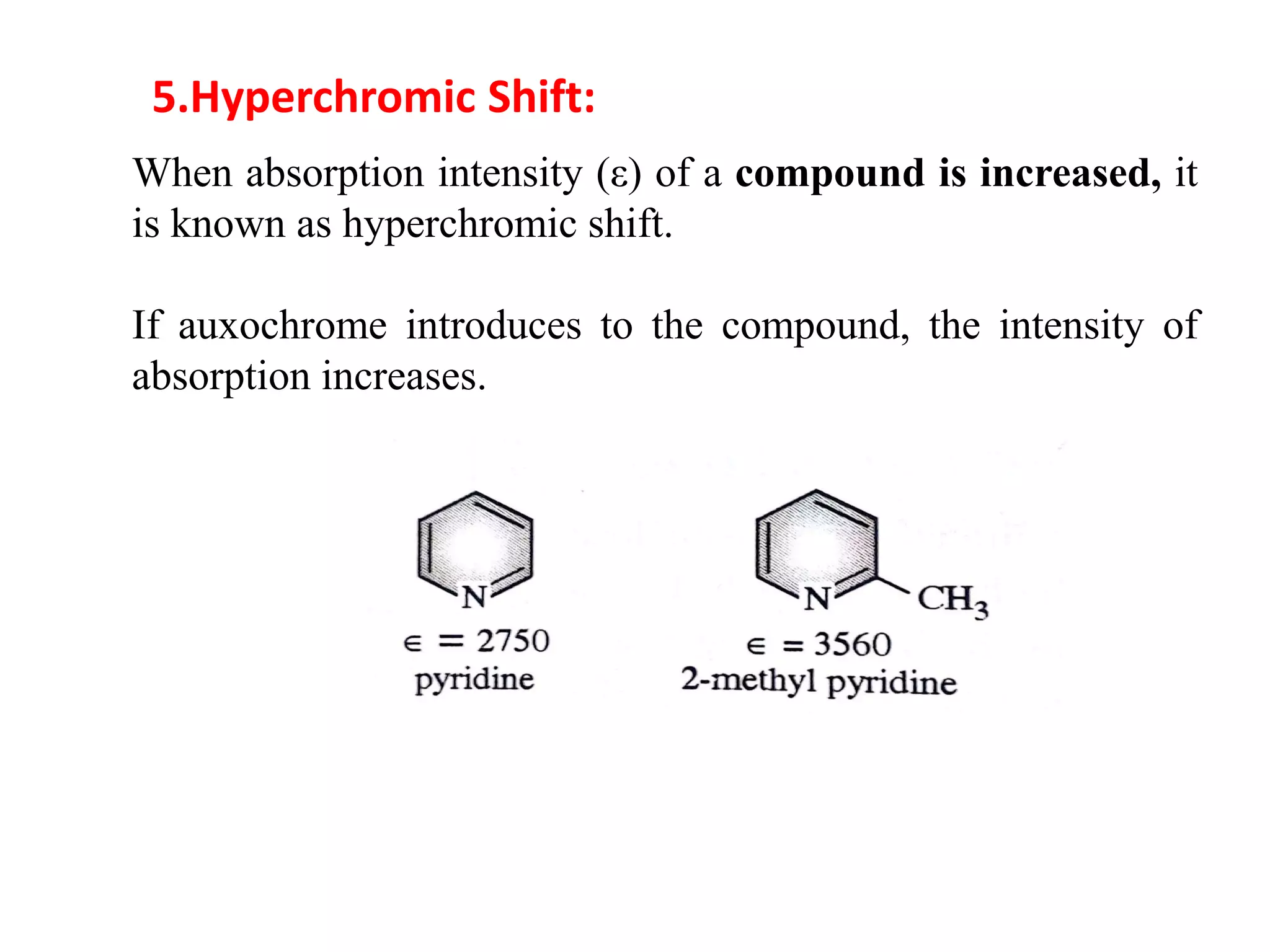
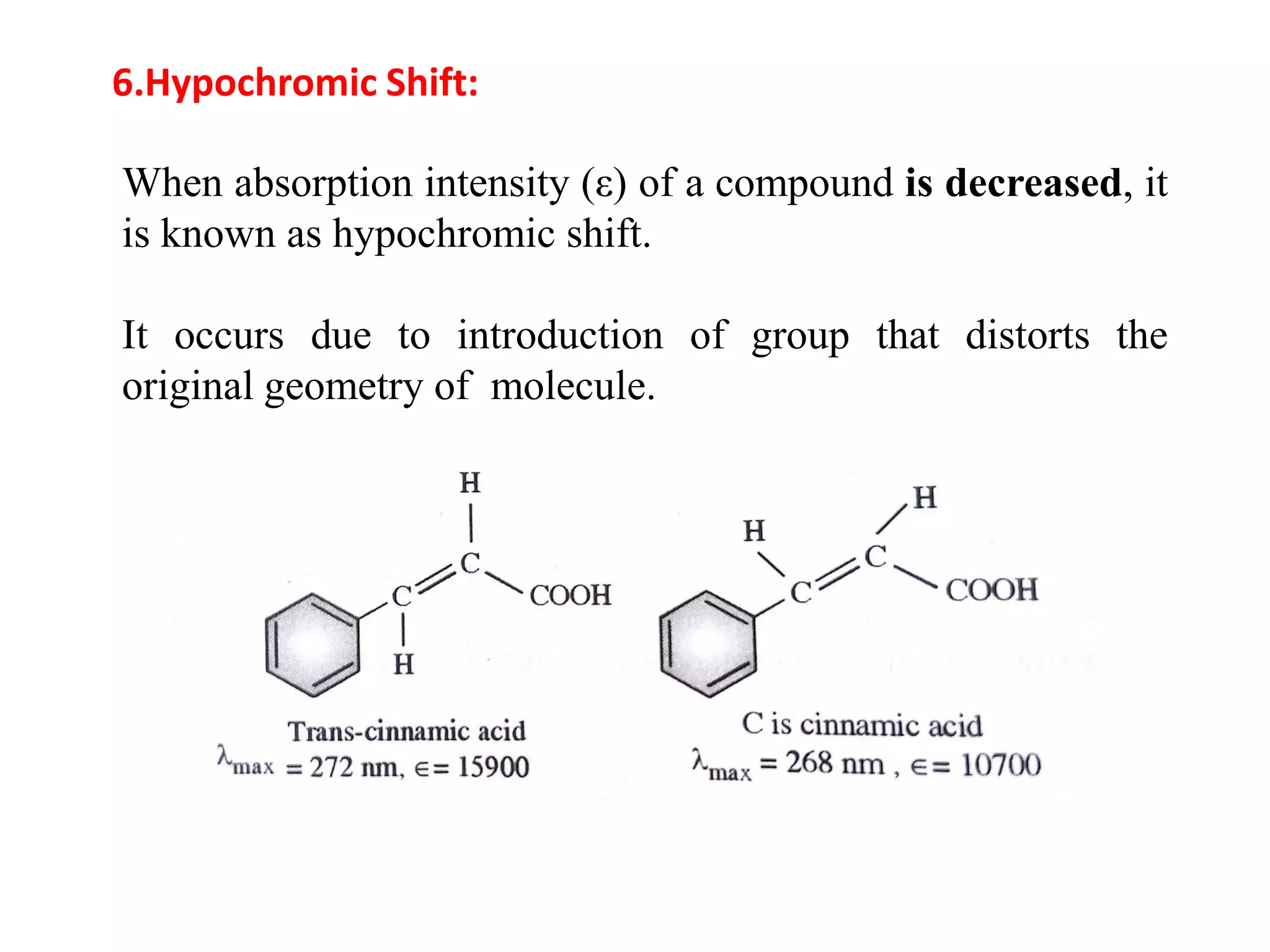
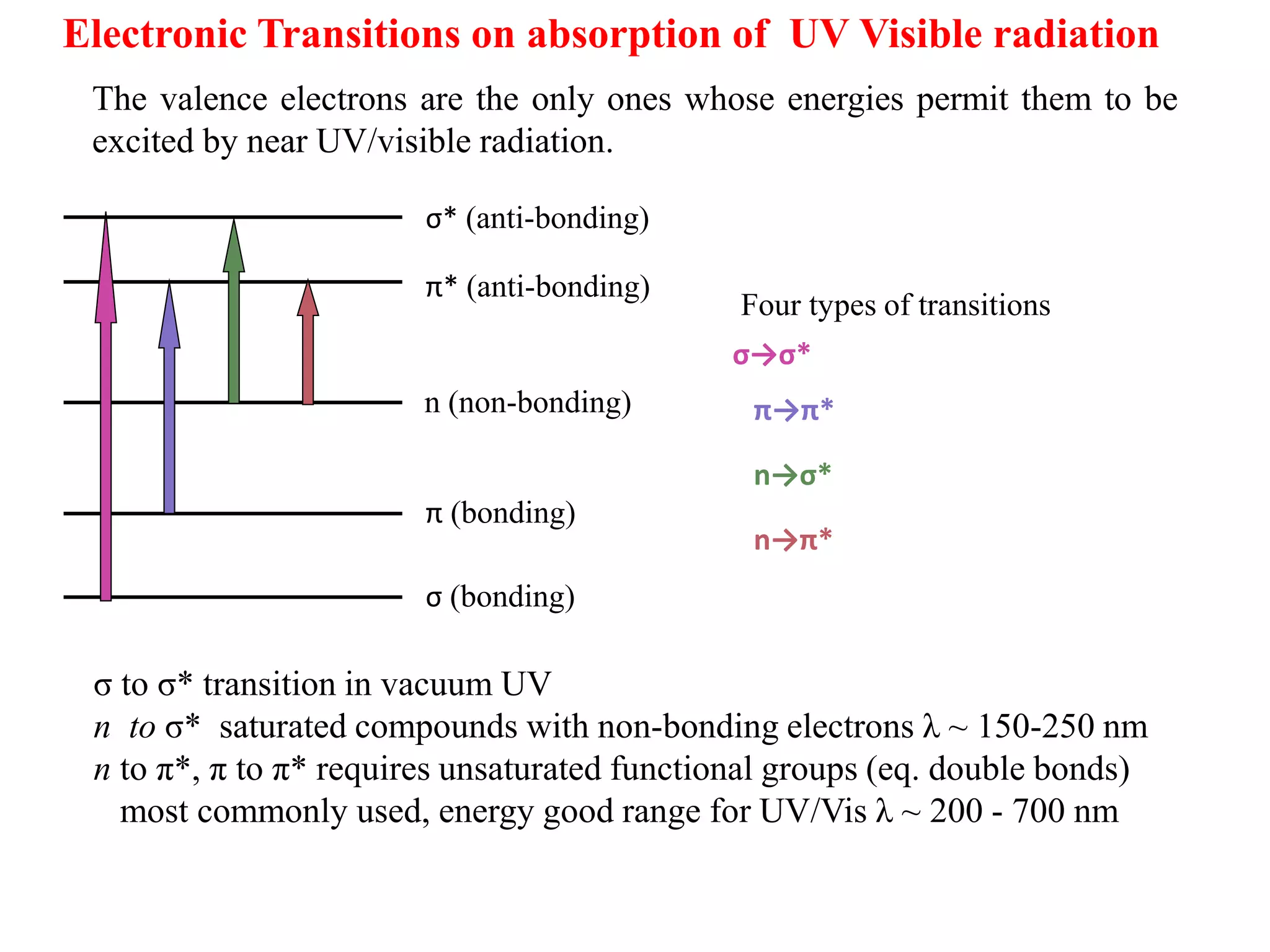
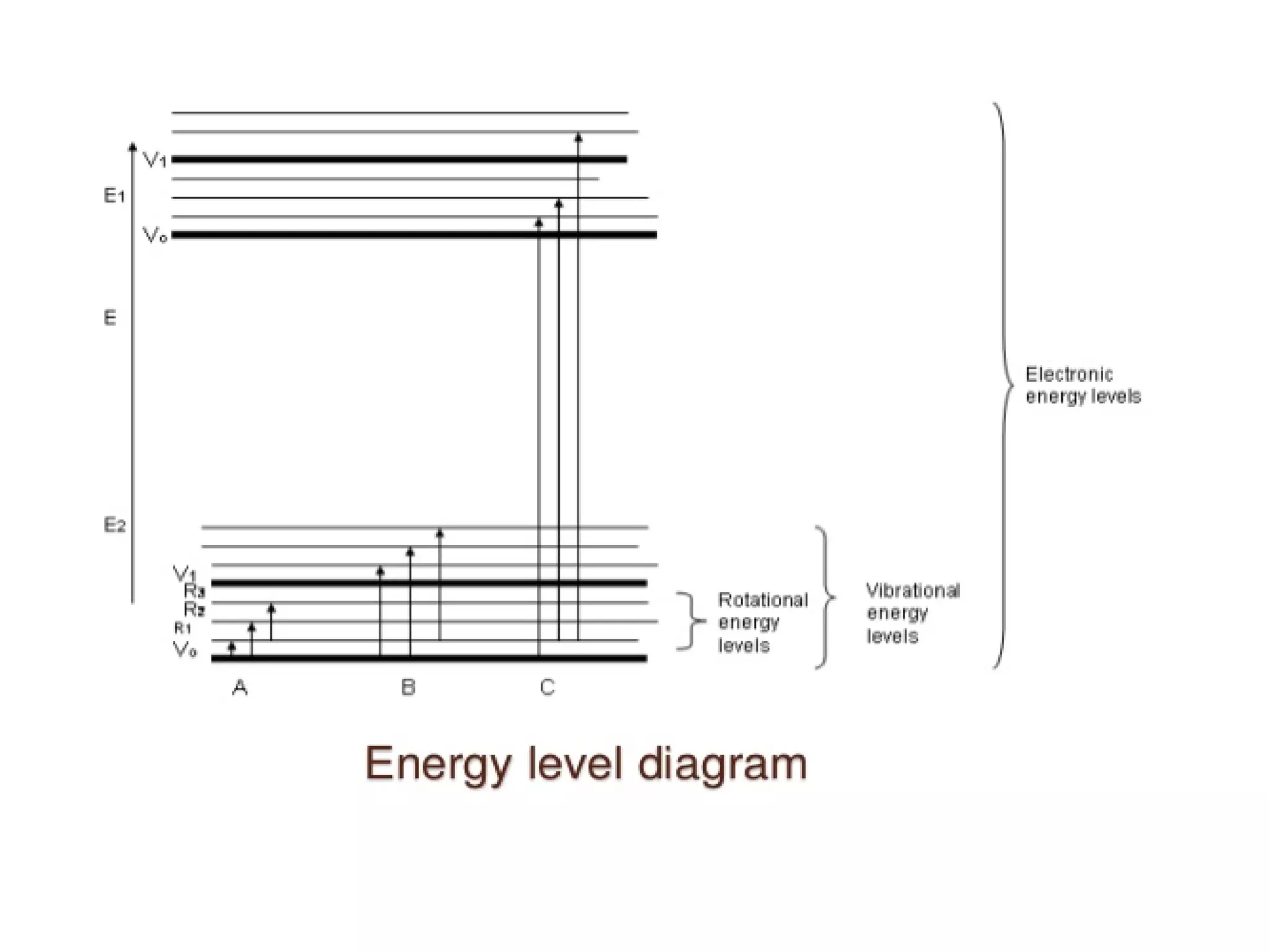
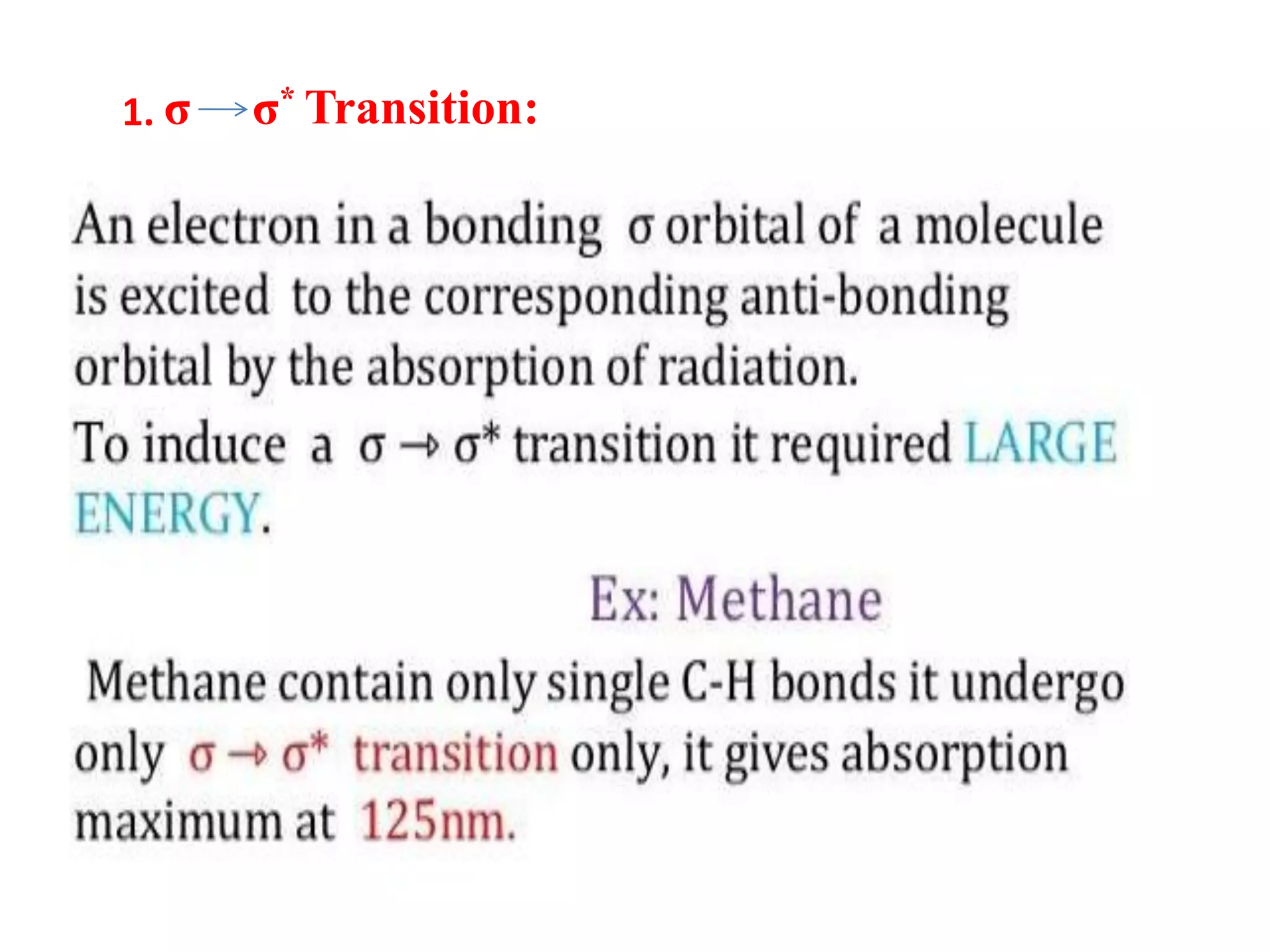
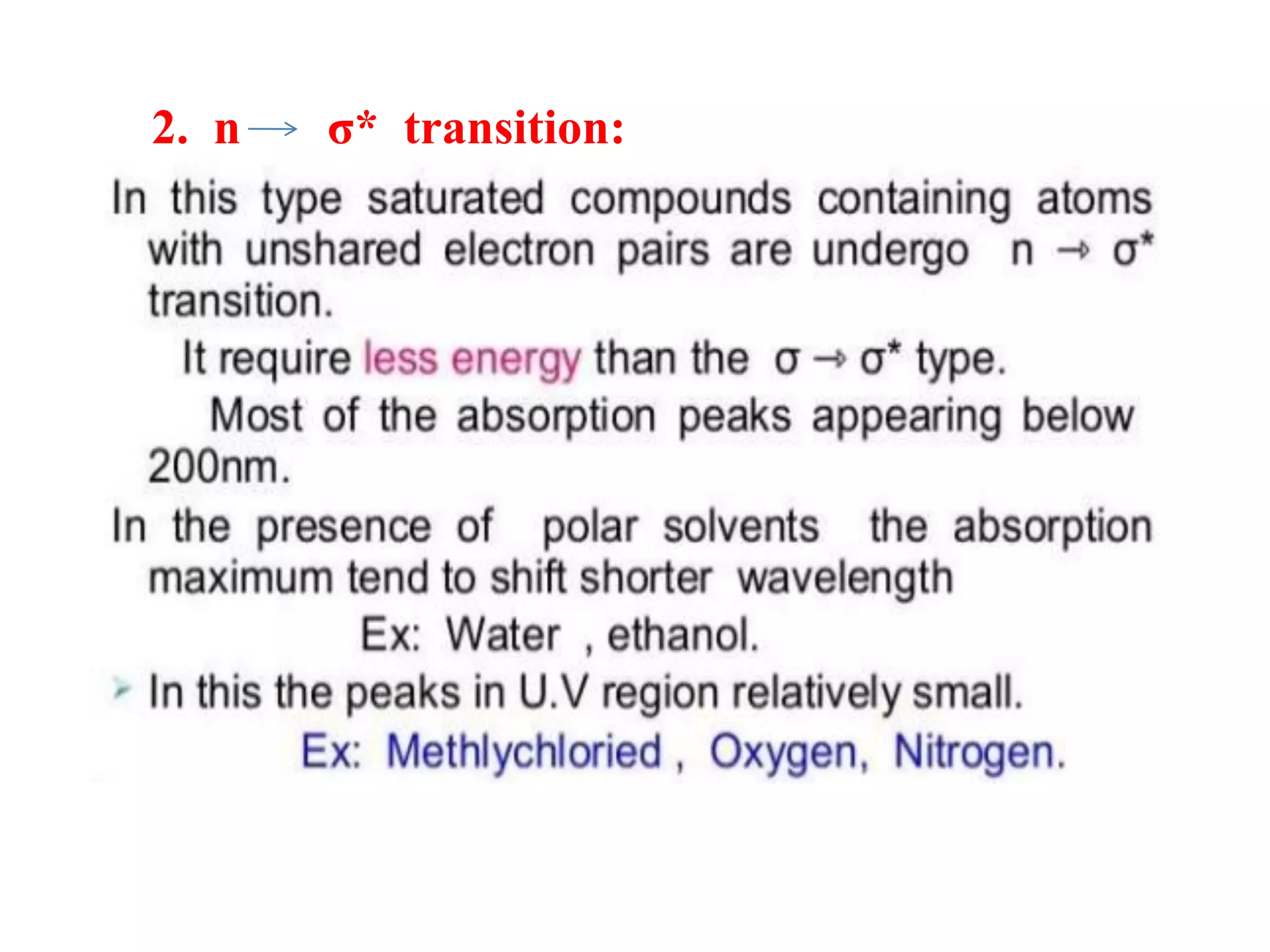
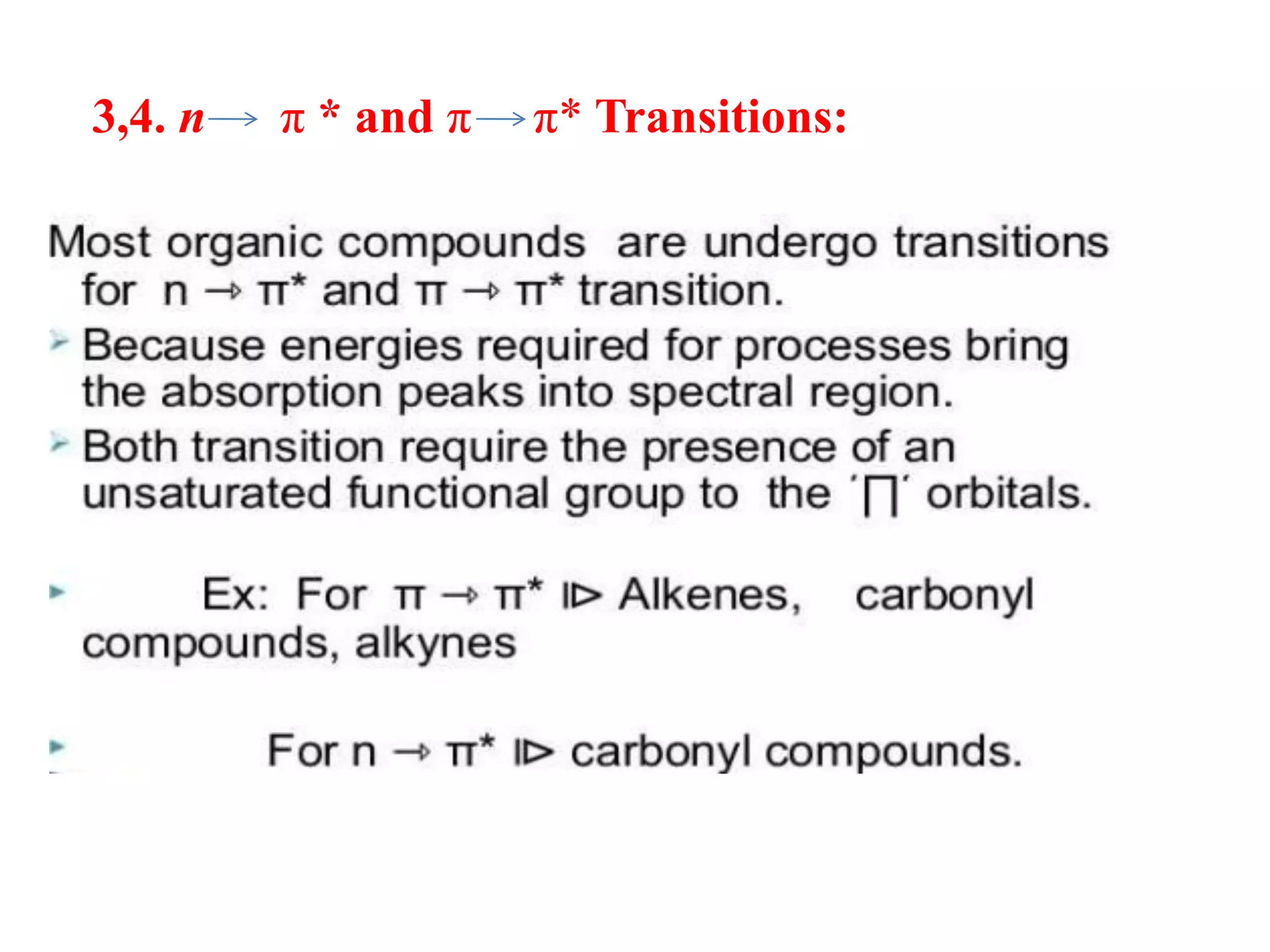
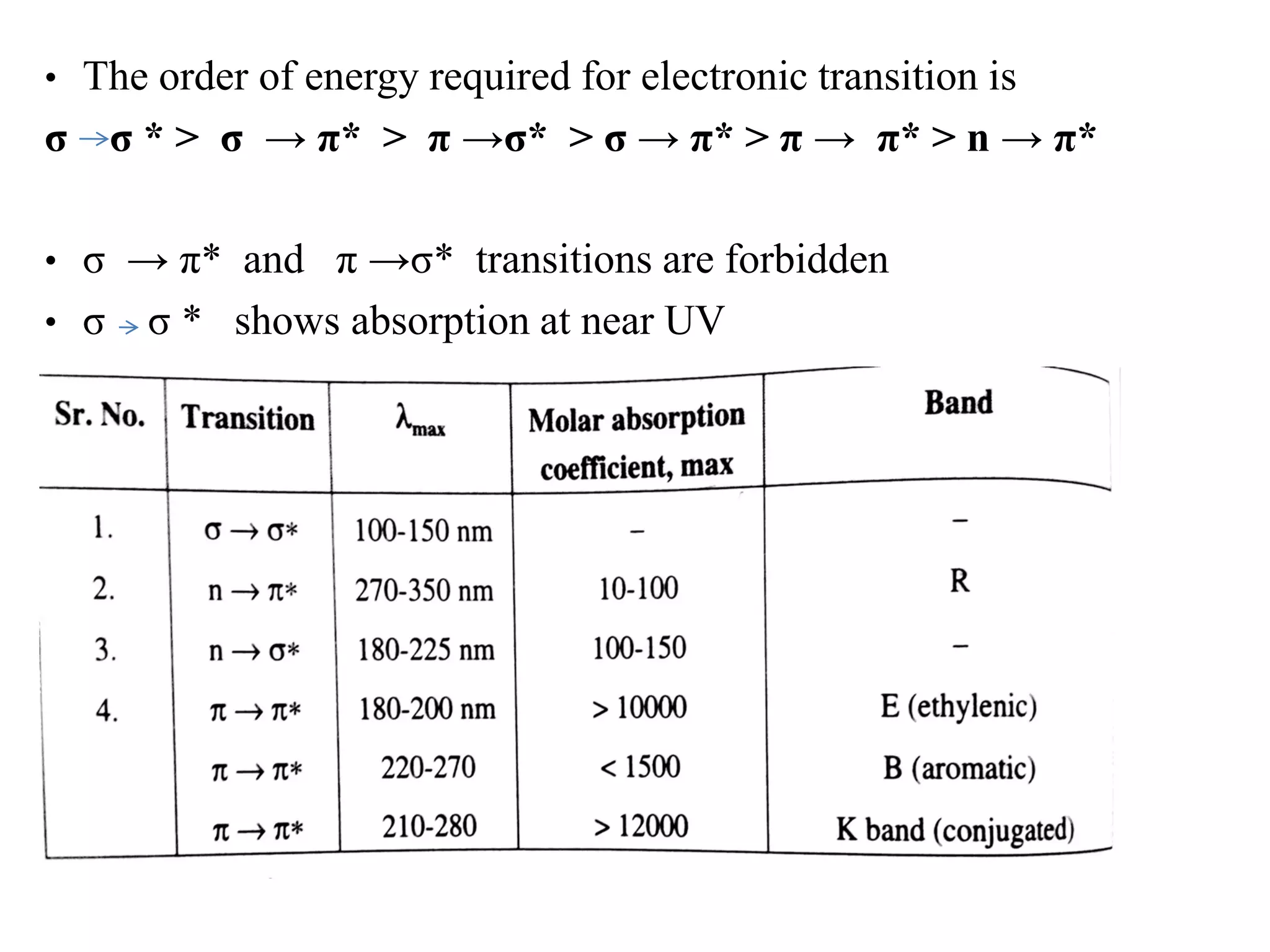
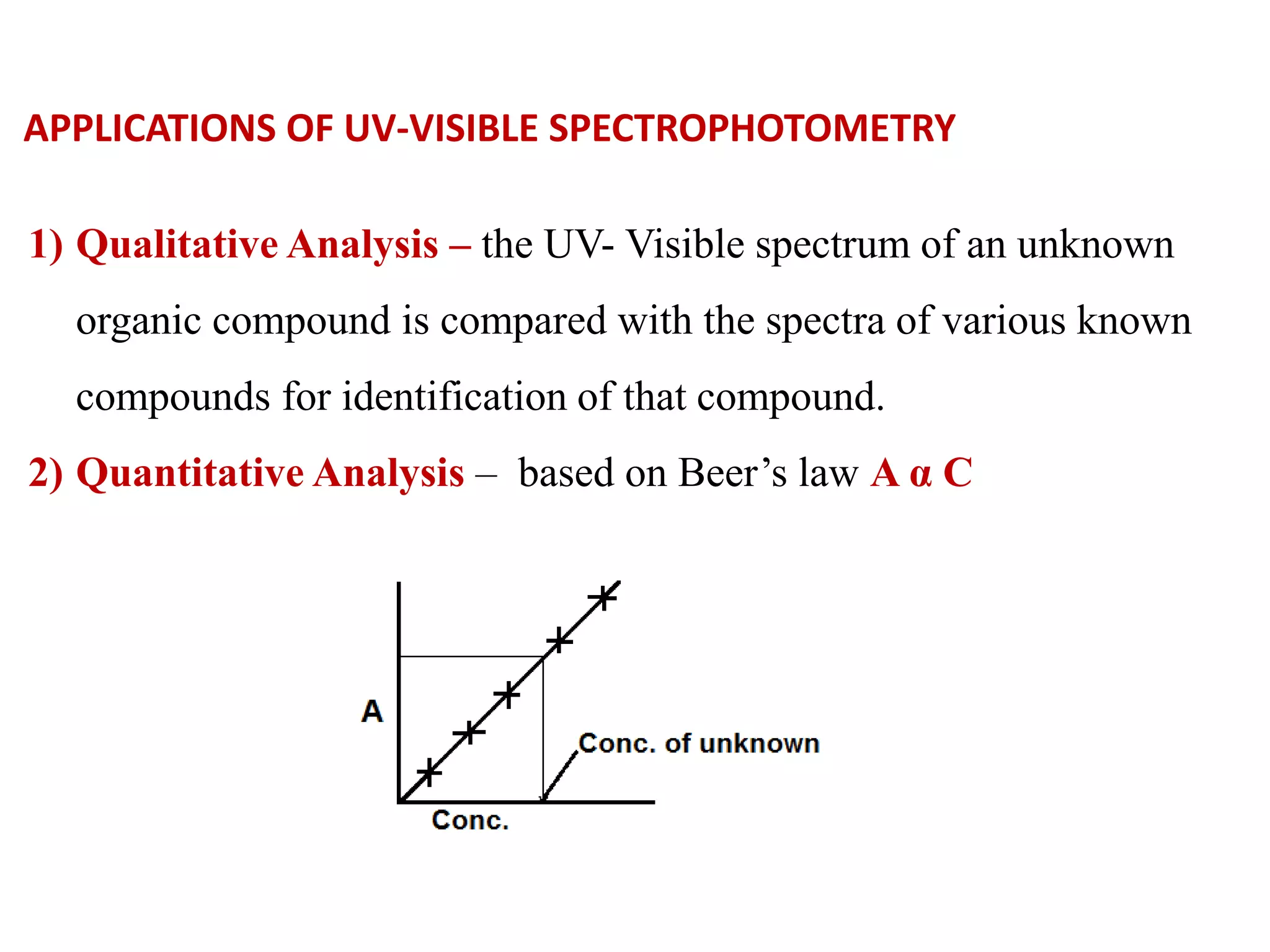
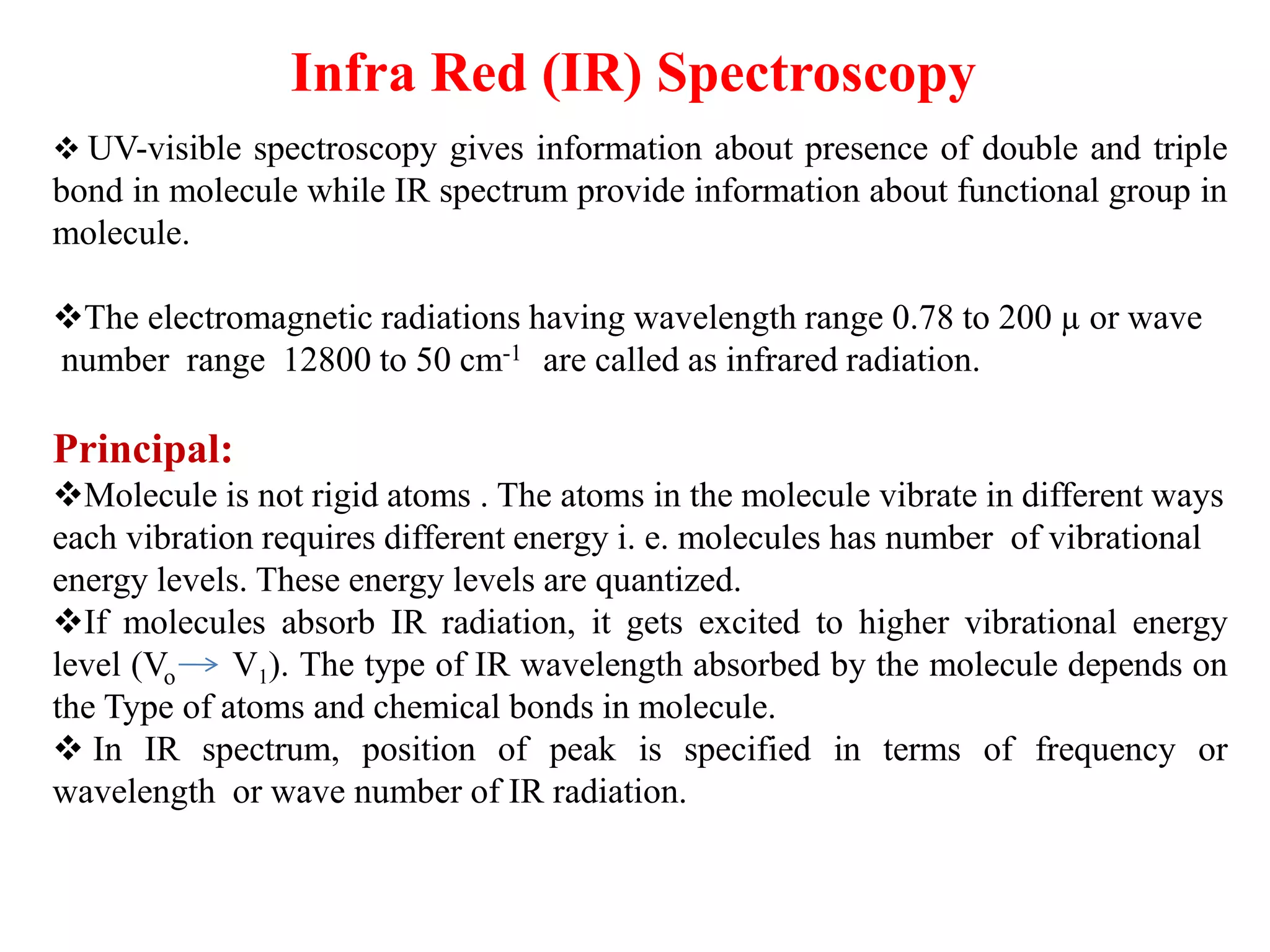
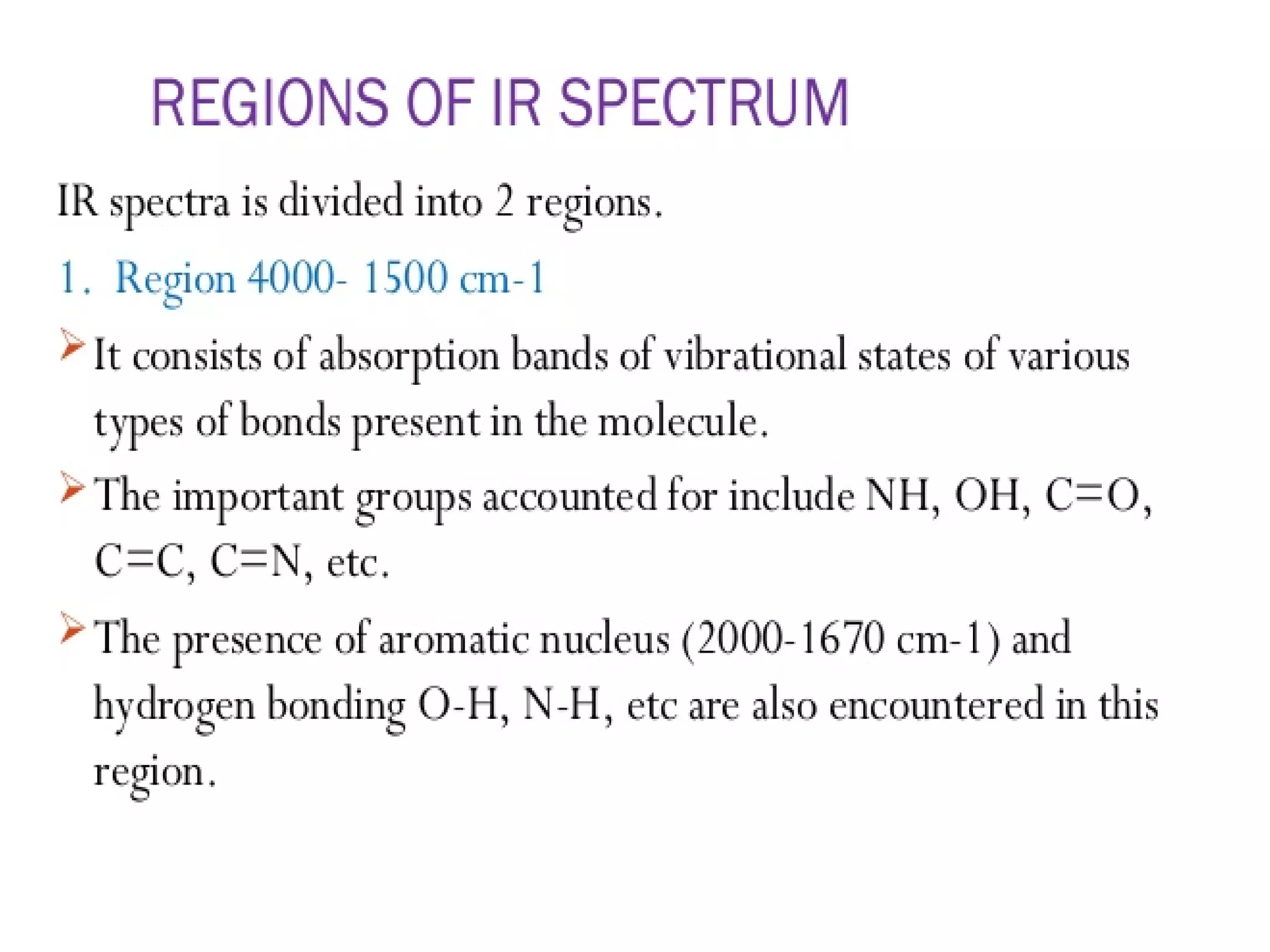
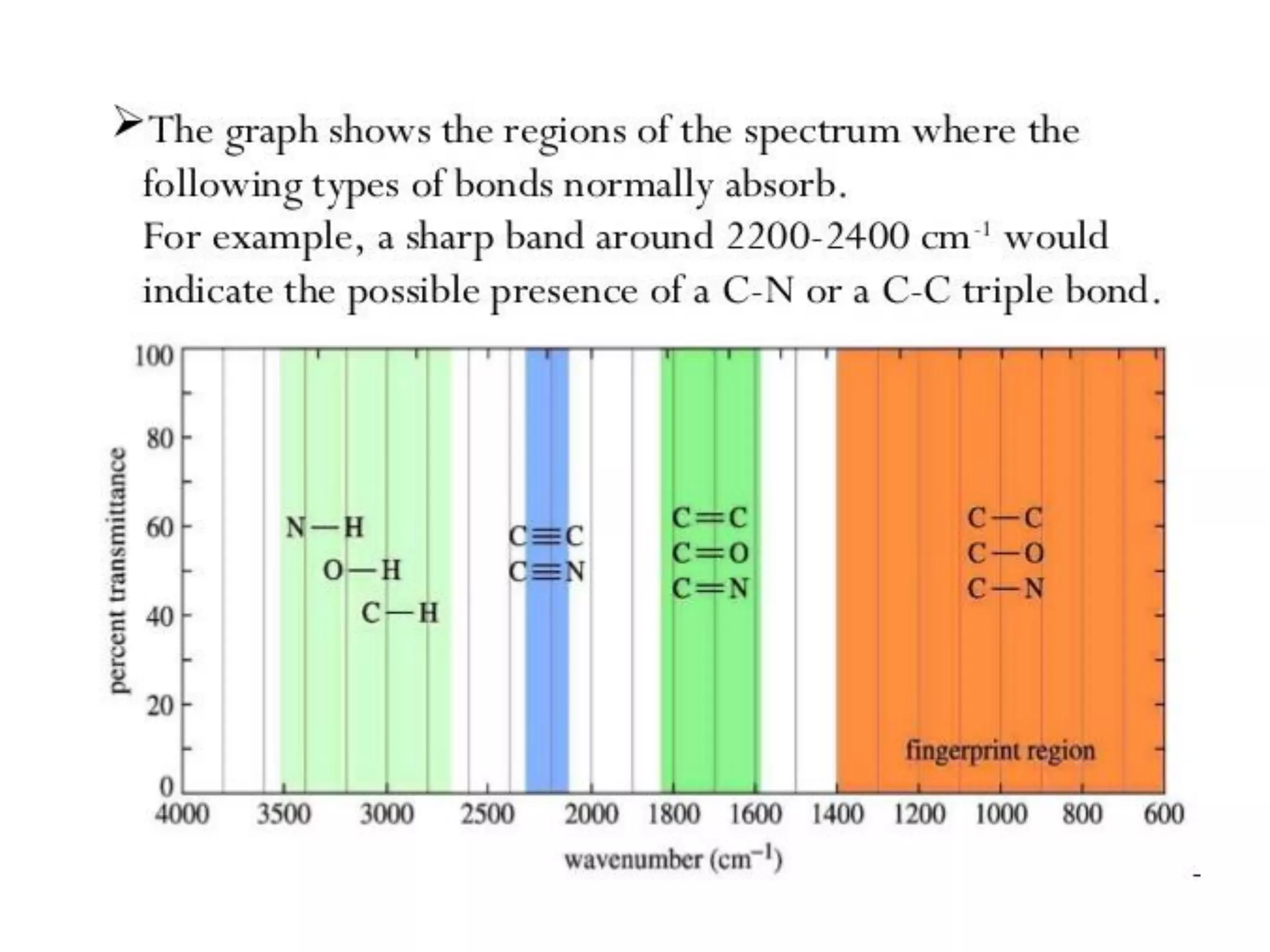
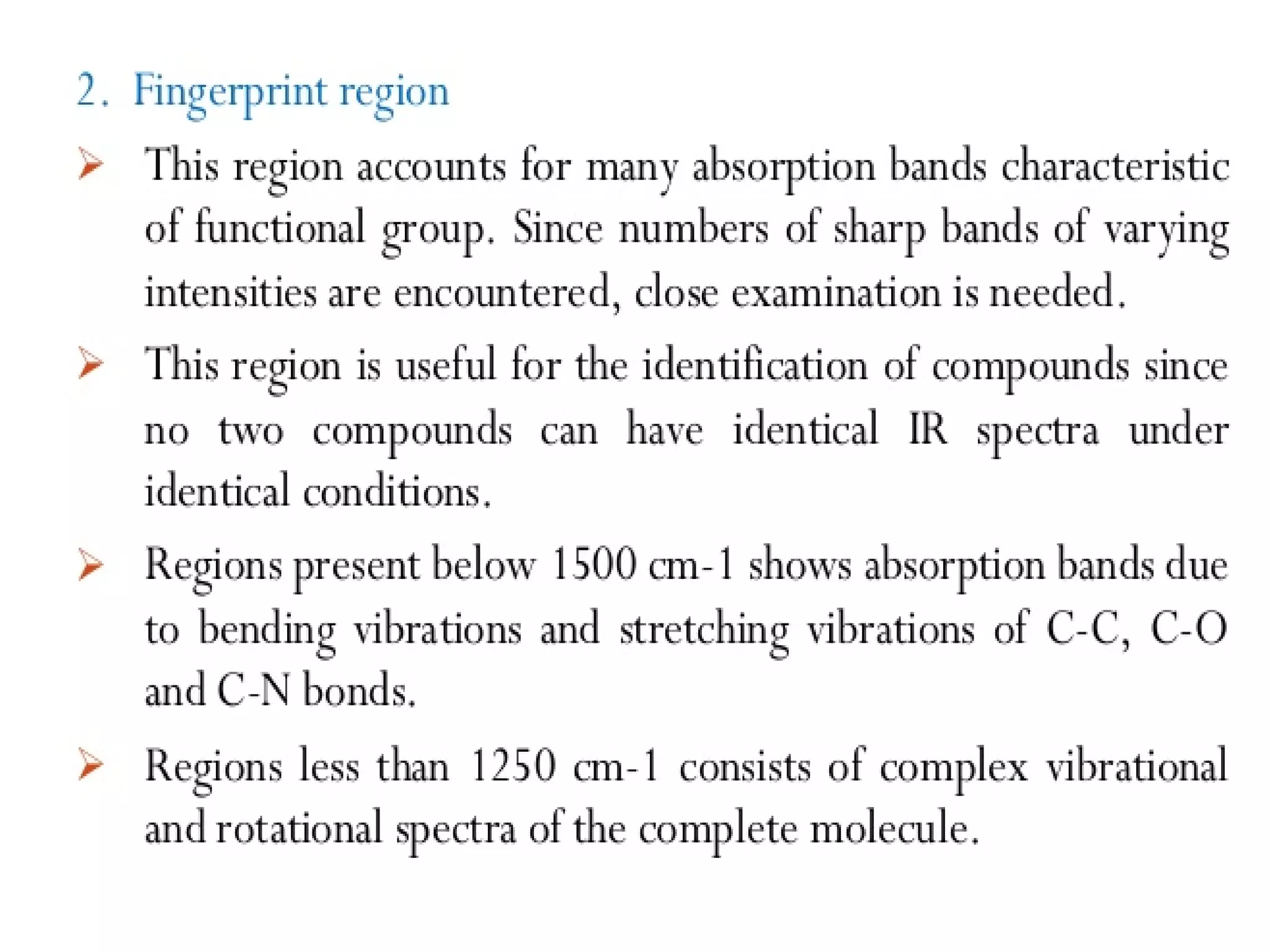
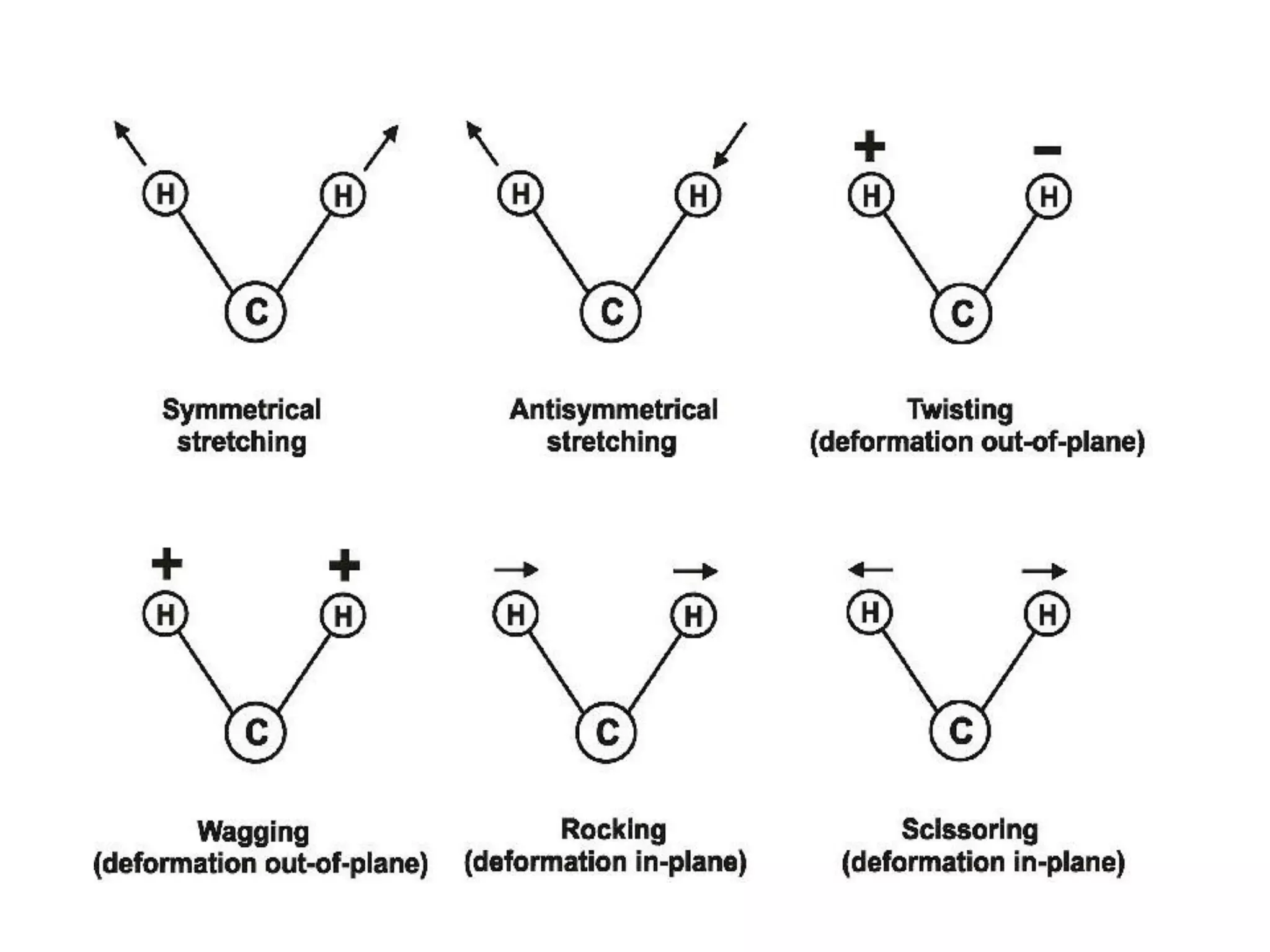
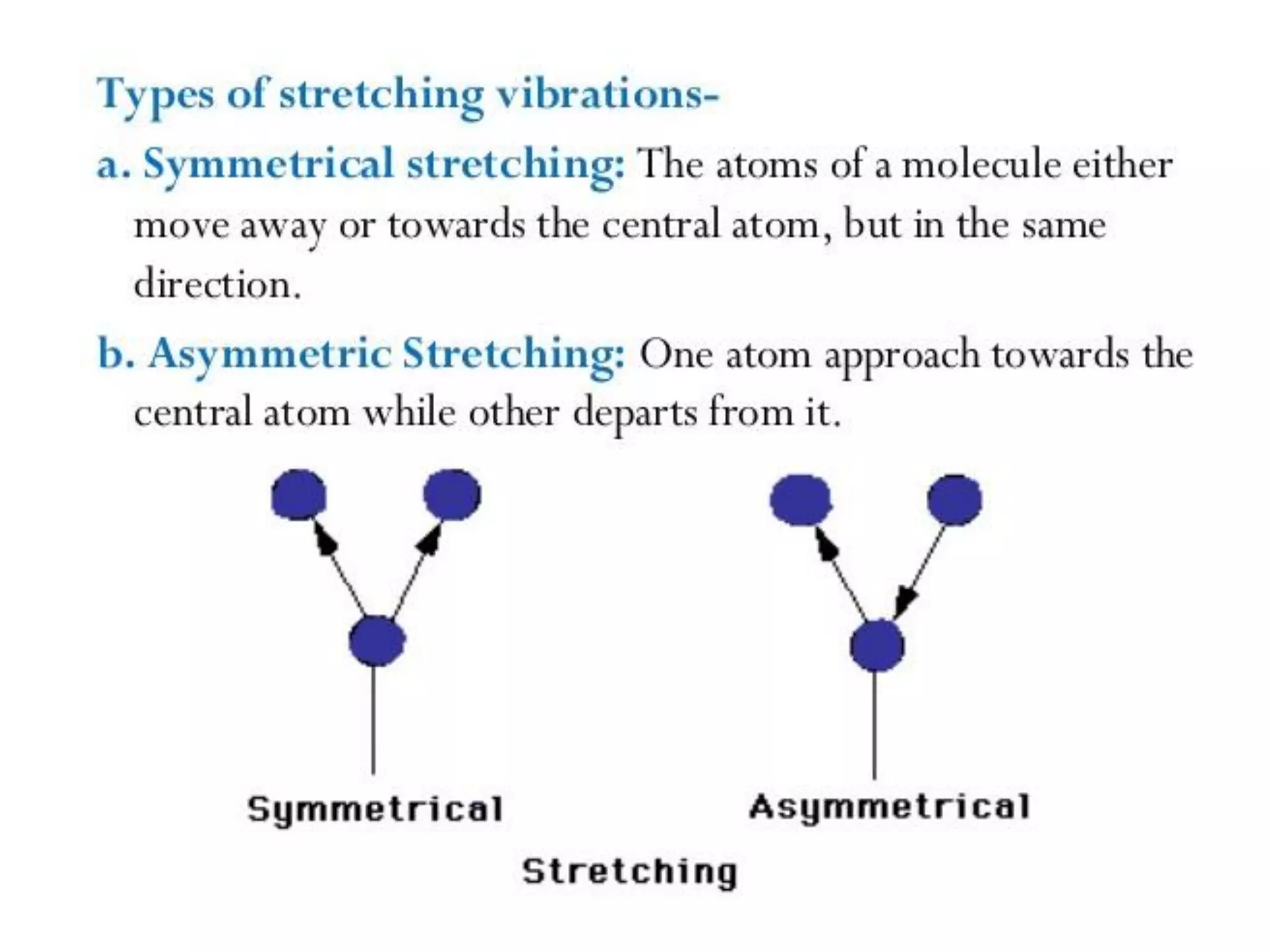
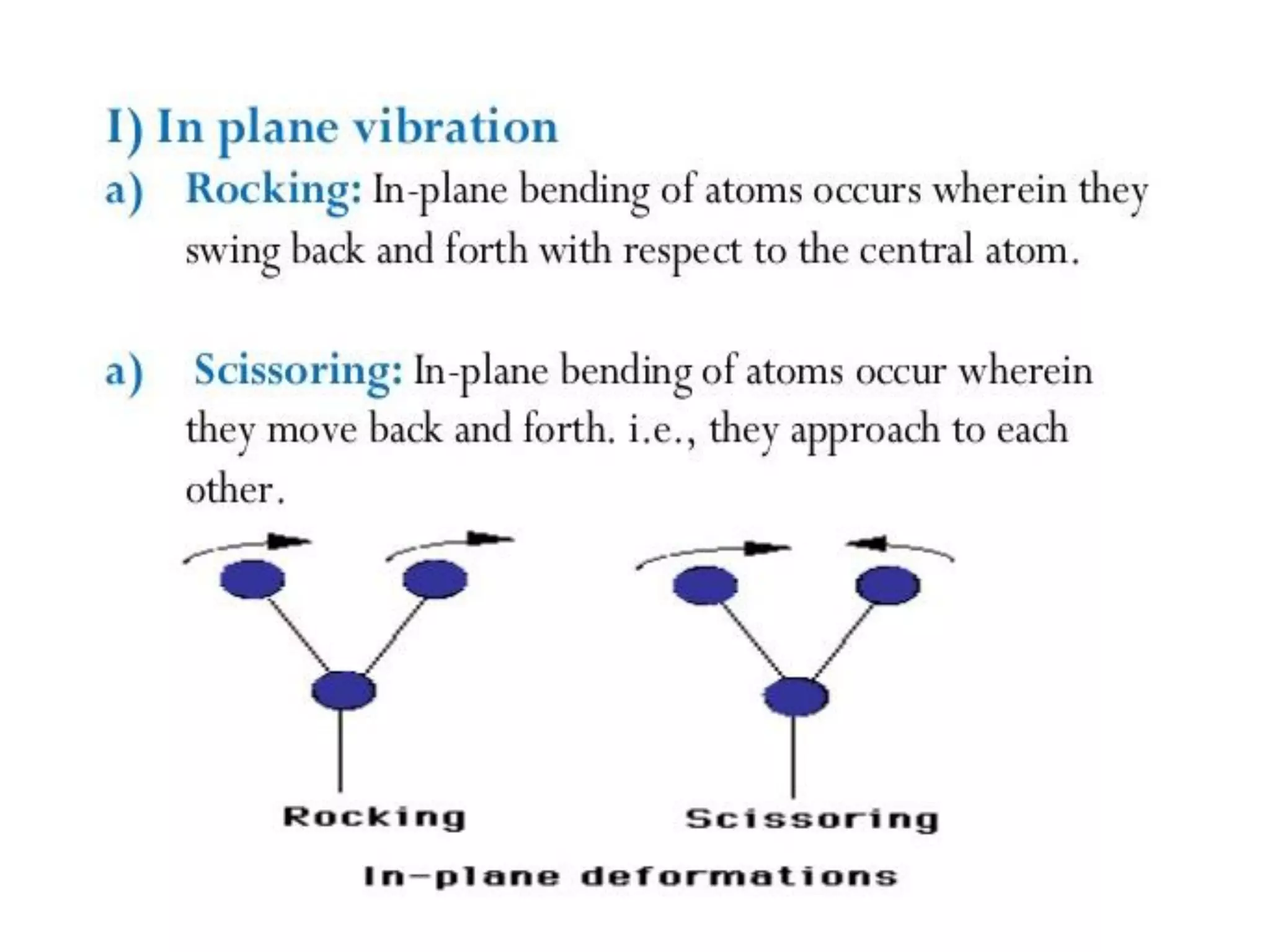
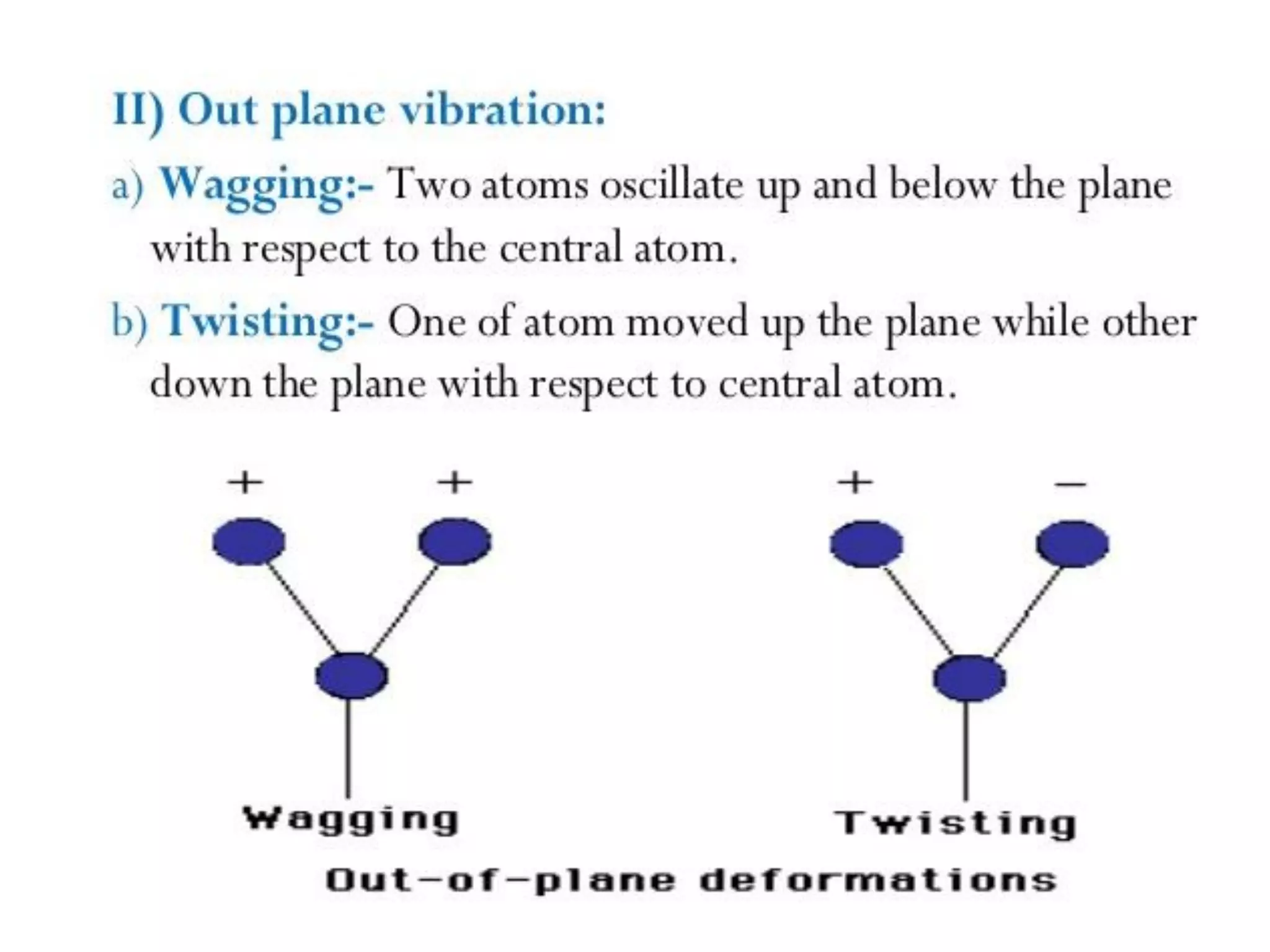
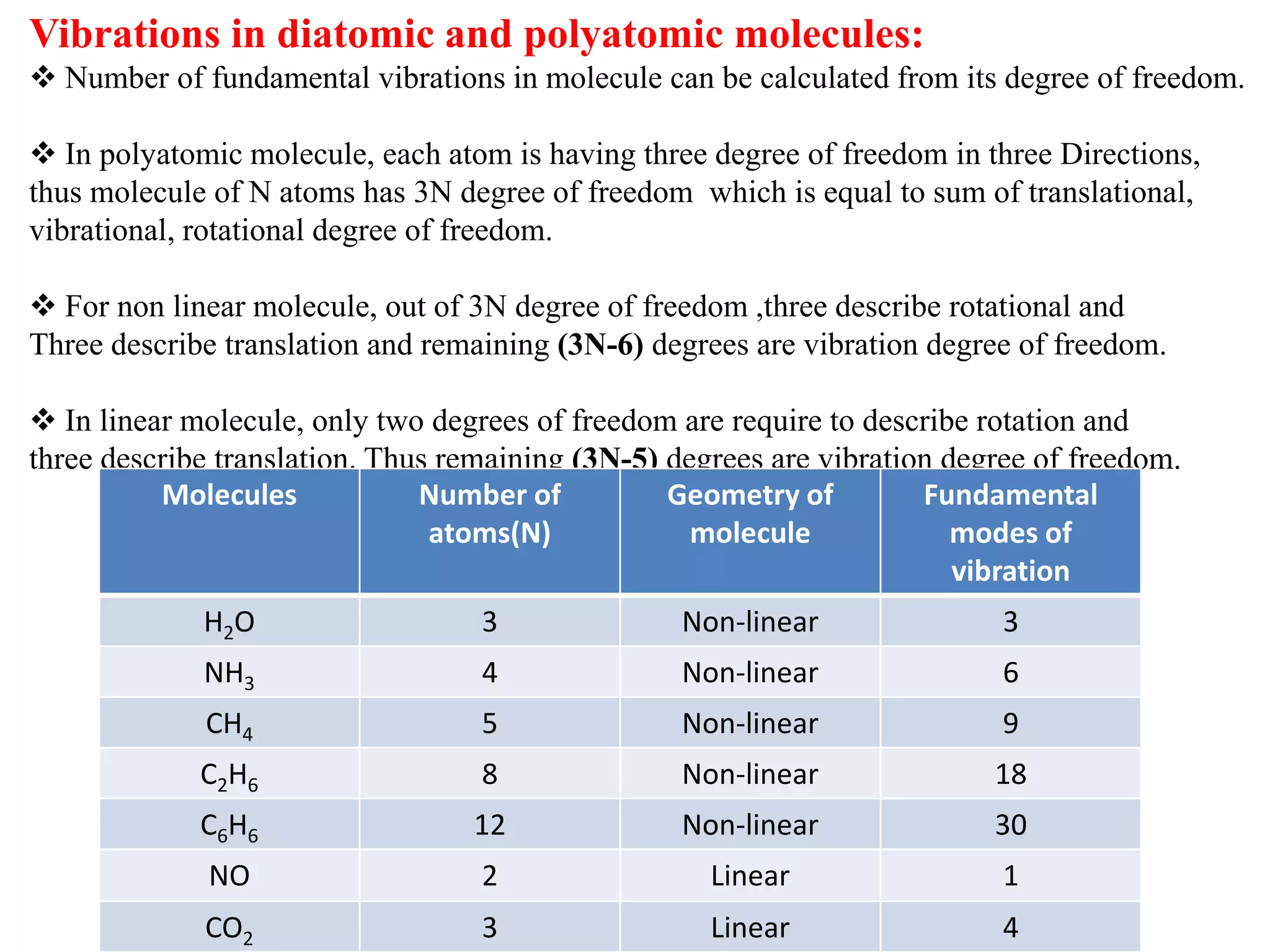
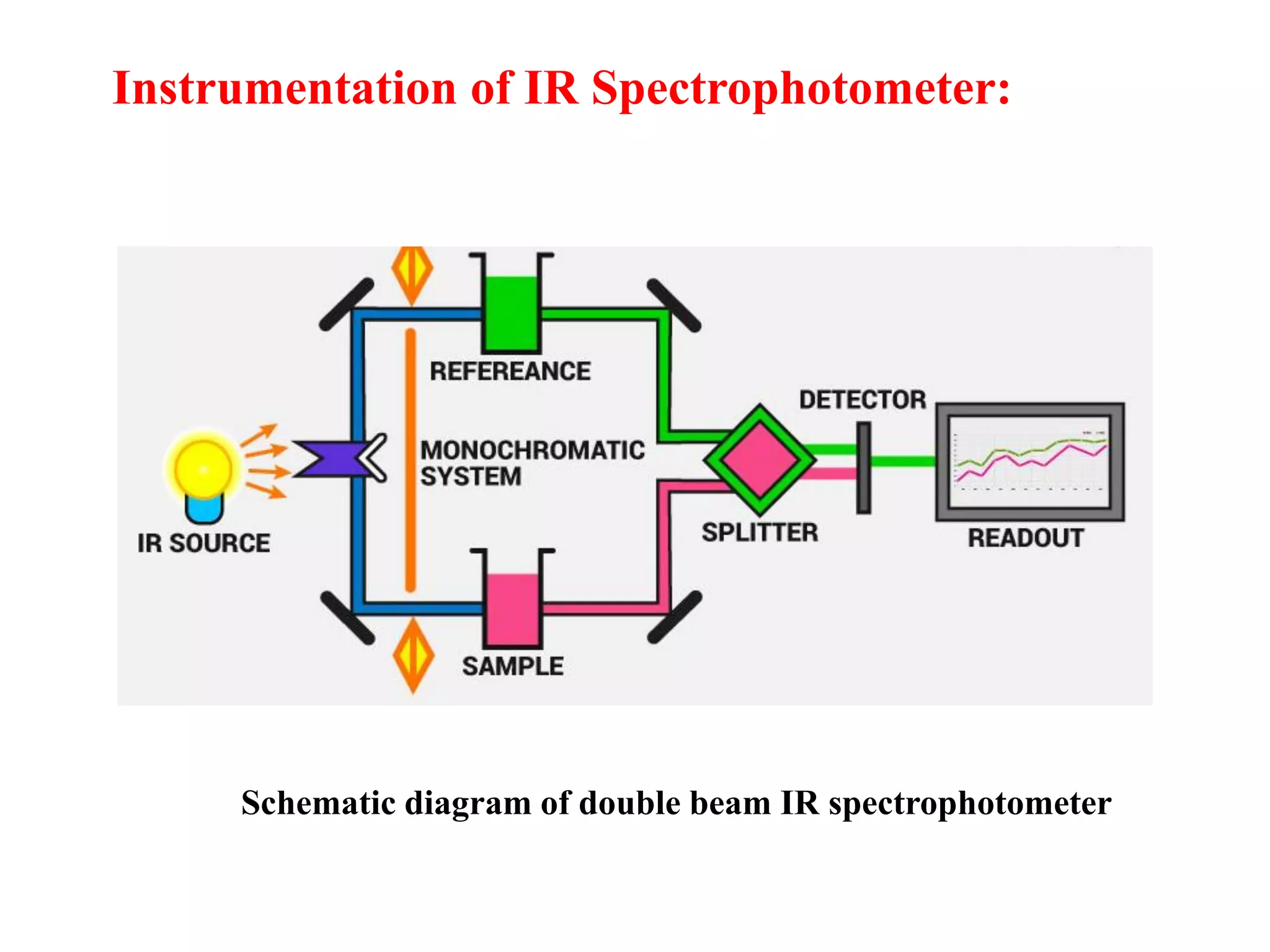
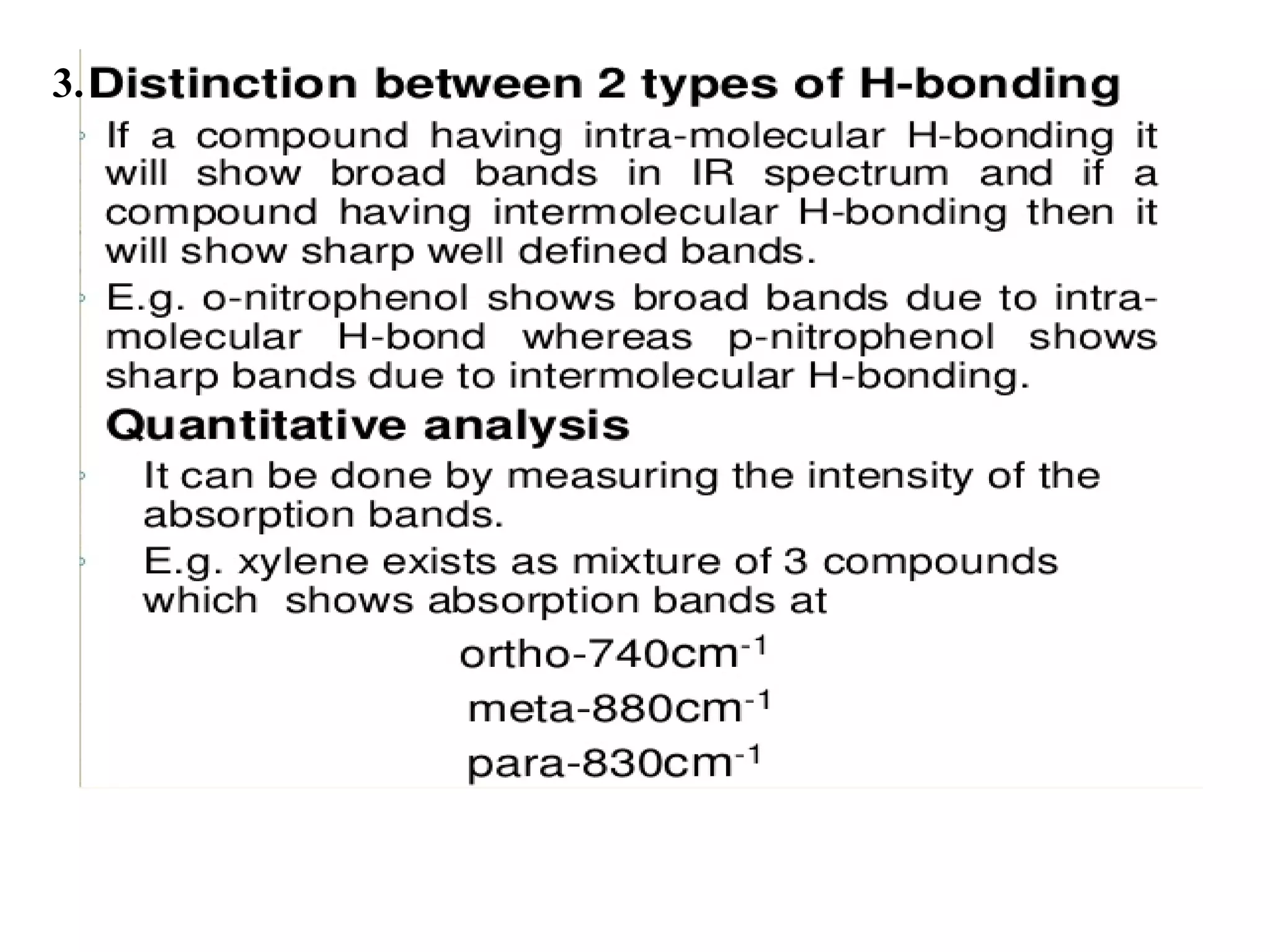
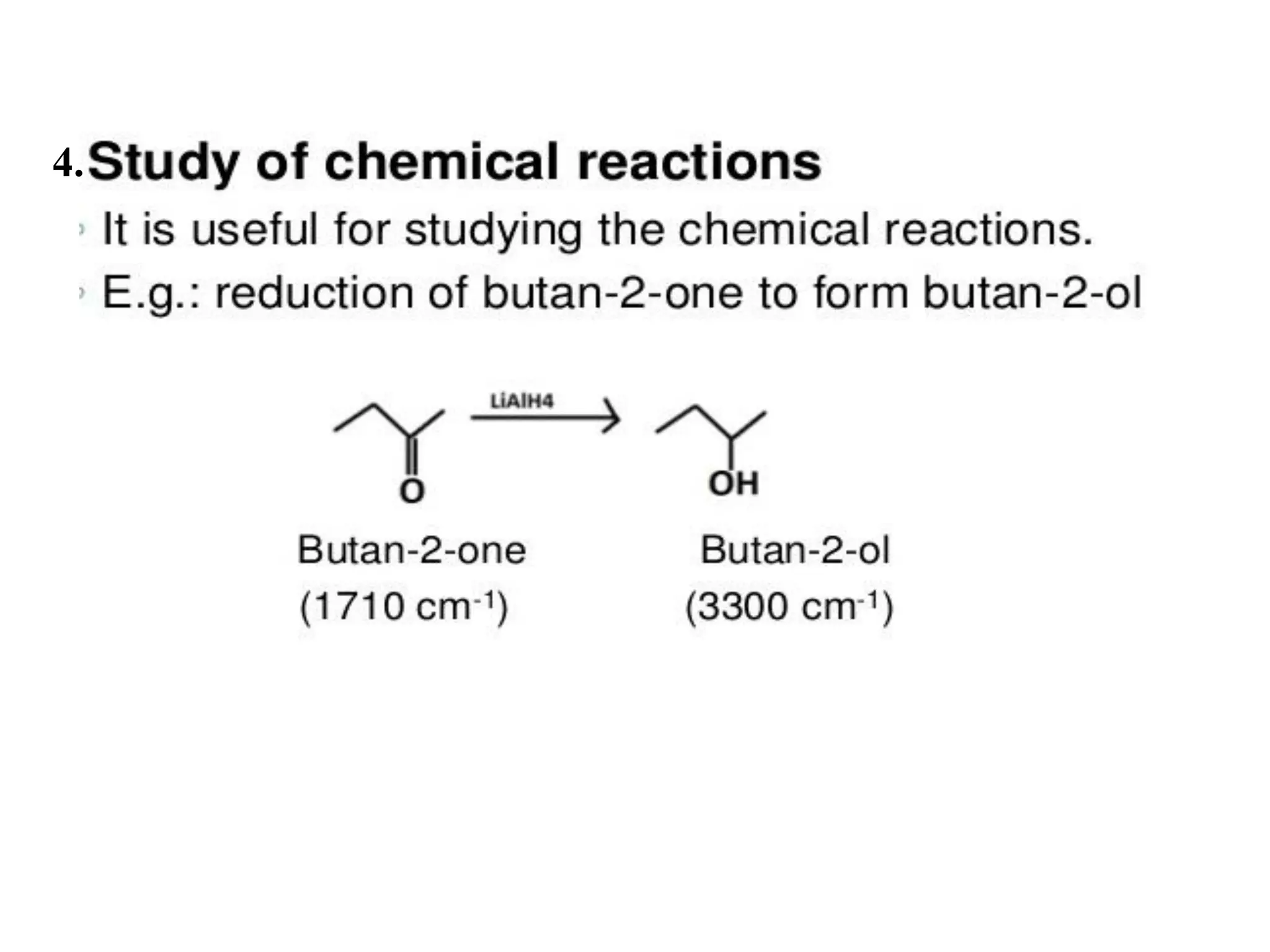
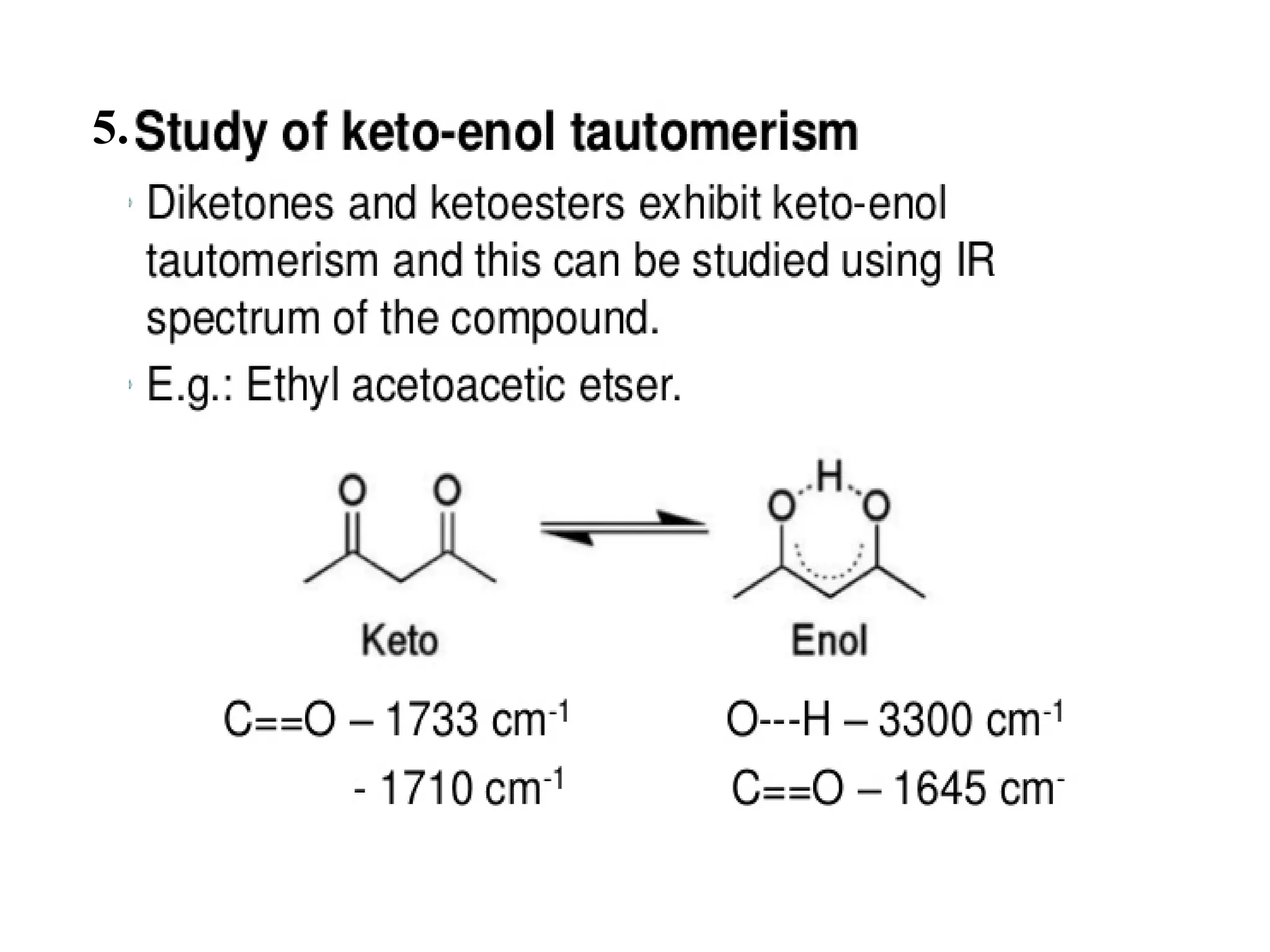
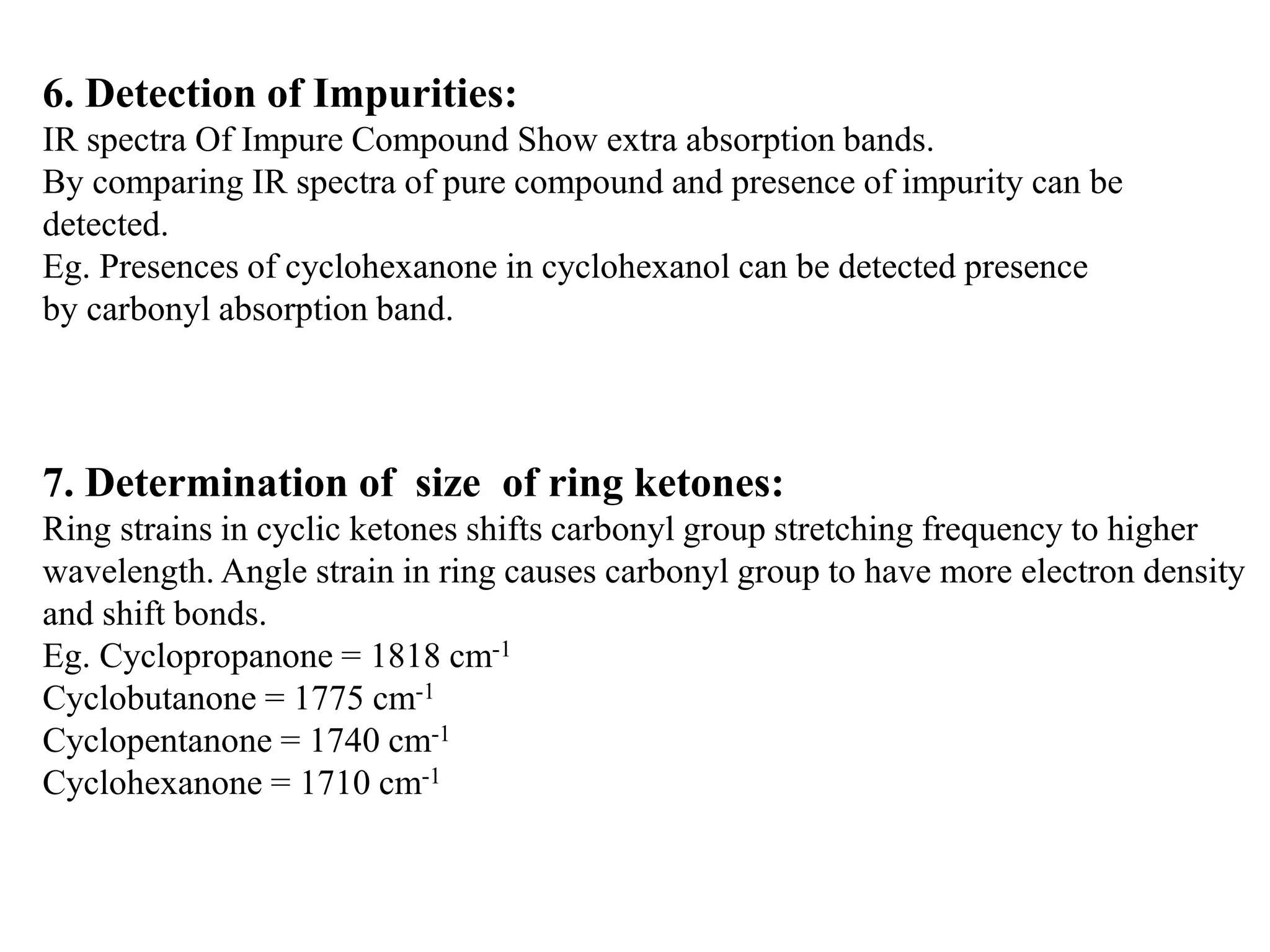
Spectroscopy is the study of interaction of electromagnetic radiation with matter. Spectroscopic techniques are based on measurement of electromagnetic radiation emitted or absorbed by a sample. The main spectroscopic techniques discussed are UV-Visible spectroscopy and Infrared (IR) spectroscopy. UV-Visible spectroscopy provides information about double and triple bonds in molecules, while IR spectroscopy provides information about functional groups. Both techniques can be used for qualitative and quantitative analysis of compounds.