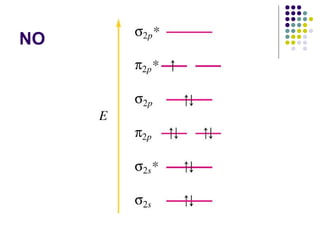
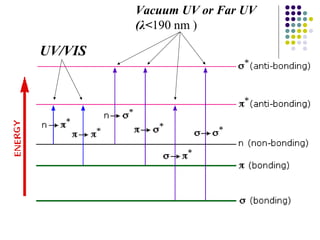
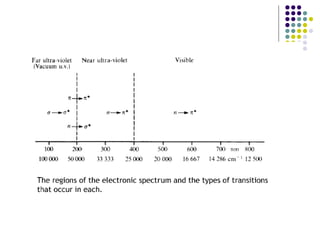
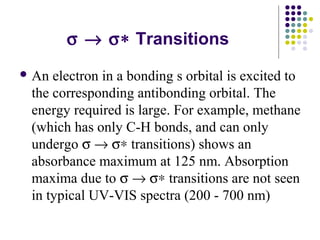
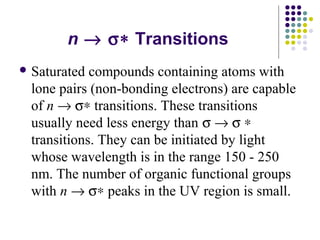
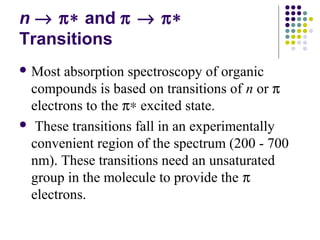
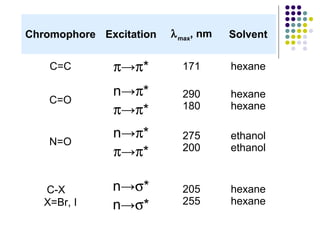
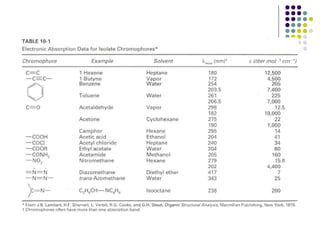
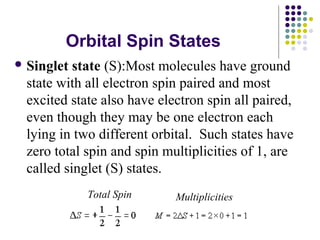
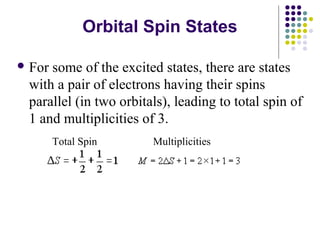
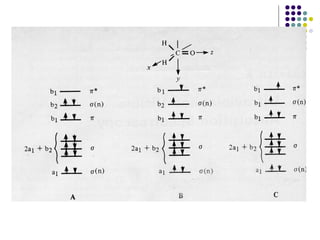
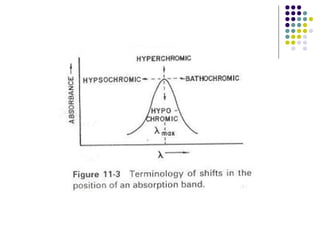
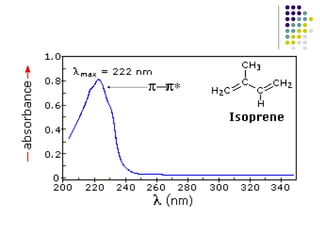
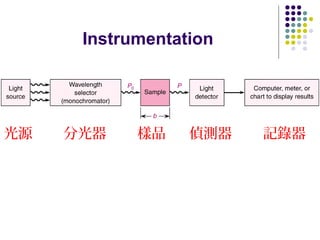
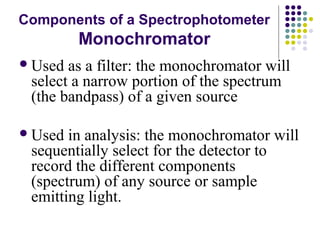
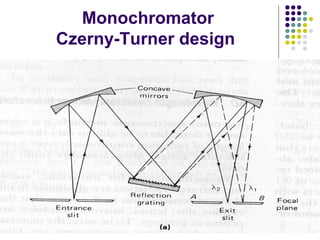
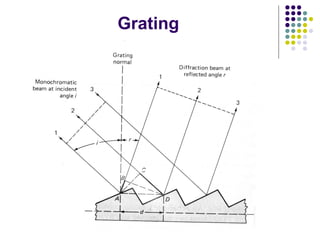
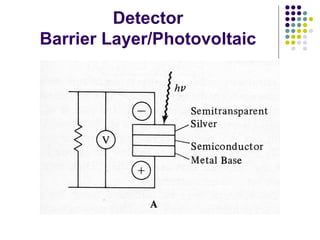
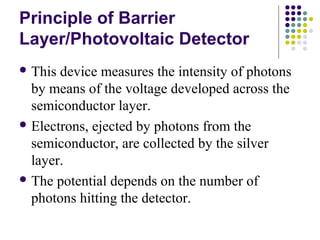
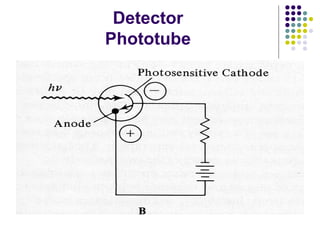
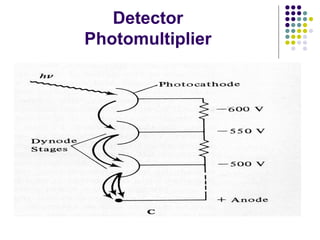
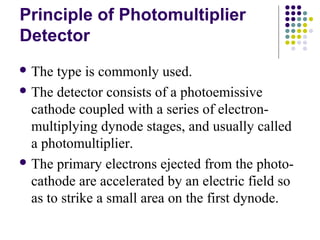
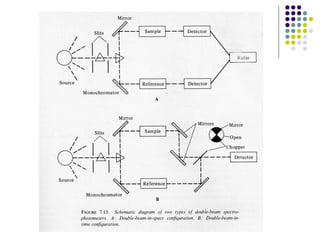
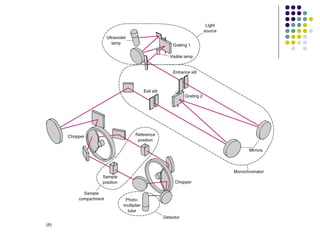
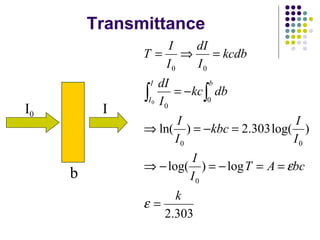
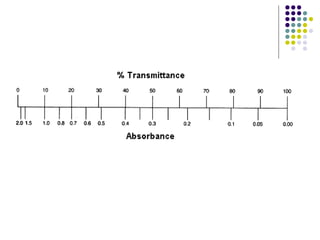
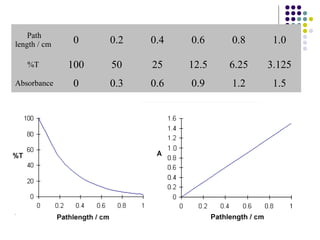
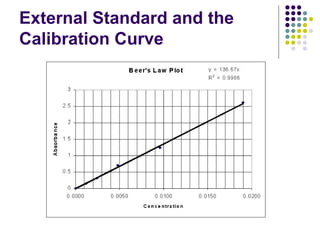
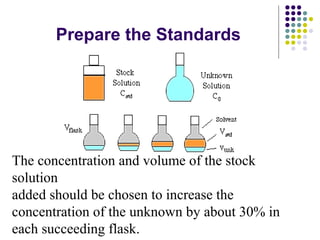
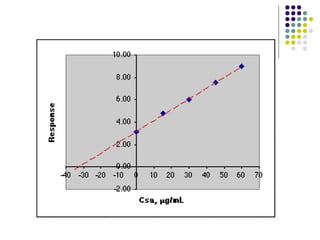
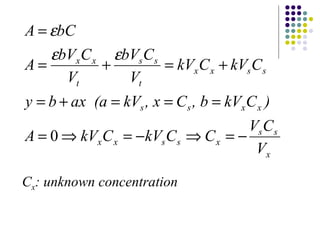
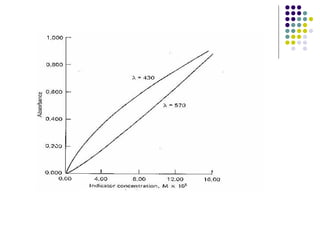
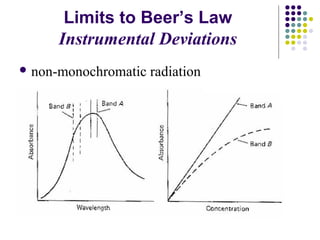
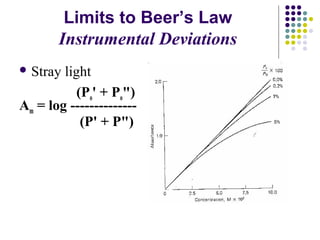
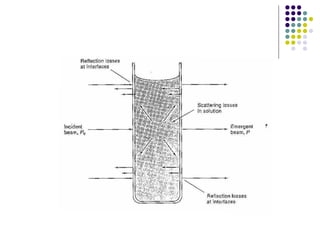
This document provides an overview of molecular spectroscopy, with a focus on visible and ultraviolet spectroscopy. It describes the electromagnetic spectrum and different types of molecular transitions. UV-Vis spectroscopy involves electronic transitions between molecular orbitals that are excited by photons in the UV-Vis range. The document discusses instrumentation for UV-Vis spectroscopy including light sources, monochromators, detectors, and single and double beam spectrometers. It also covers quantitative analysis using Beer's Law and limitations to Beer's Law. Applications of UV-Vis spectroscopy include structure determination and quantitative analysis of absorbing species containing p, s, and n electrons.